Datasheet is currently unavailable. Try again or CONTACT US
HeLa Cell Nuclear Extract Doxorubicin Stimulated
W09-001-A82
200 µg
Liquid (sterile filtered)
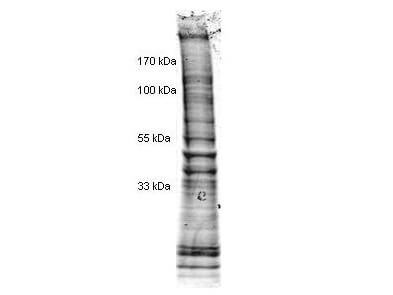
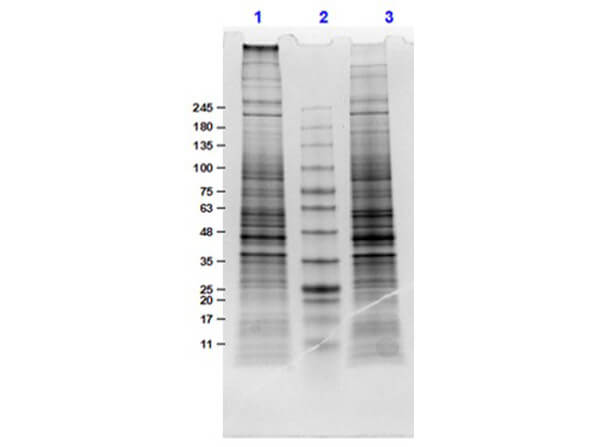
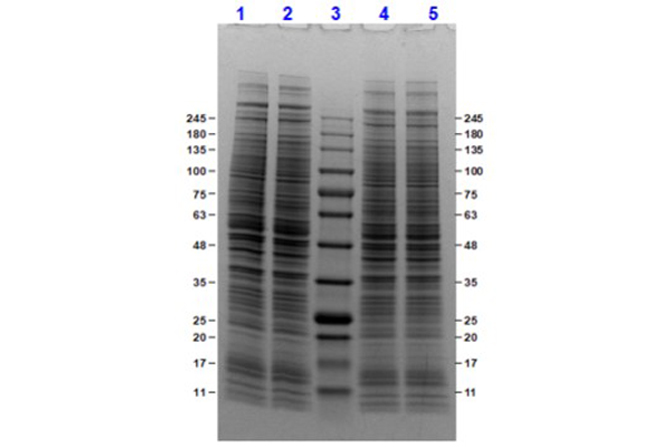
SDS-PAGE
Human
Shipping info:
$50.00 to US & $70.00 to Canada for most products. Final costs are calculated at checkout.
Product Details
HeLa Cell Nuclear Extract - Doxorubicin Stimulated - W09-001-A82
HeLa Cell Nuclear Extract Doxorubicin Stimulated, HeLa Nuclear Lysate Doxorubicin Stimulated, HeLa Doxorubicin Stimulated Nuclear Extract, HeLa Doxorubicin Stimulated Nuclear Lysate
Human
Target Details
The cells were grown in DMEM supplemented with 10% FBS (Fetal Bovine Serum). Cells were treated with 0.5 µg/ml Doxorubicin for 2 h. The lysate was prepared by first washing the cells in PBS. Washed cells were then incubated on ice in lysis buffer containing 10 mM HEPES, 60 mM KCl, 1.0 mM EDTA, 0.075% (v/v) NP40 and 1.0 mM DTT, pH 7.6. Protein integrity is ensured using a cocktail of protease inhibitors with broad specificity for the inhibition of aspartic, cysteine, and serine proteases as well as aminopeptidases (0.1 mM AEBSF HCl, 0.08 µM Aprotinin, 5 µM Bestatin, 1.5 µM E-64, 2 µM Leupeptin Hemisulfate and 1 µM Pepstatin A). Nuclei were then collected and washed in lysis buffer minus detergent. Nuclei were lysed by vortexing in extraction buffer containing 20 mM Tris-Cl, 1.5 mM MgCl2, 0.42 M NaCl, 0.2 mM EDTA, and 25% (v/v) glycerol, pH 8.0, supplemented with protease inhibitors (see above). The lysate was clarified by centrifugation. Protein concentration was determined by Lowry assay using a commercially available kit. The protein concentration was adjusted to 2.0 mg/ml and then an equal volume of 2X SDS-PAGE sample buffer was added.
Application Details
SDS-PAGE
W09-001-A82 has been tested by SDS-PAGE. Ready-to-use nuclear extracts are especially prepared as positive controls for separation by SDS-PAGE and subsequent western blot analysis. Nuclear extracts are supplied in denaturing buffer without dissociating agents. Heat nuclear extract to 95° C for 5 minutes and rapidly cool. If dissociating conditions are desired add reducing agent prior to heating. The recommended loading volume per lane is 10-30 µL depending on the size format of your gel.
Cell Line Data
HeLa - Human epidermoid carcinoma
Nuclear Extract
Doxorubicin
Tissue Culture
Doxorubicin (0.5 µg/ml)
Formulation
1.0 mg/mL by BCA assay
1X SDS-PAGE Sample Buffer (62.5 mM Tris HCl, 2% SDS, 10% Glycerol and 0.005% bromophenol blue, pH 6.8)
None
10% (v/v) Glycerol
Shipping & Handling
Dry Ice
Store vial at -70° C or COLDER. For extended storage, aliquot contents to minimize freeze/thaw cycles.
Expiration date is three (3) months from date of receipt.
Ready-to-use nuclear extracts produced by Rockland Immunochemicals are derived from cell lines or tissues using highly refined extraction protocols to ensure exceptionally high quality, protein integrity and lot-to-lot reproducibility. All extracts are tested by SDS-PAGE using 4-20% gradient gels and immunoblot analysis using antibodies to key cell signaling components to confirm the presence of both high molecular weight and low molecular weight proteins.
No test method can provide total assurance that the hepatitis B virus, hepatitis C virus, human immunodeficiency virus, or any other infectious agents are absent. Thus, all blood products, including purified proteins derived from human blood sources, should be handled at Biosafety Level 2 as recommended by the CDC\NIH manual entitled Biosafety in Microbiological and Biomedical Laboratories for potentially infectious human serum, blood specimens or proteins derived from same. Source material for the human blood product supplied to your facility has been tested for the detection of HIV antibody, Hepatitis B surface antigen, antibody to Hepatitis C, HIV 1 antigen(s), antibody to HTLV - I/II, and syphilis by FDA guidelines. All units were found to be non-reactive/negative for these tests. All human blood source material is collected in FDA licensed centers and is tested with FDA approved test kits.; This product is for research use only and is not intended for therapeutic or diagnostic applications. Please contact a technical service representative for more information. All products of animal origin manufactured by Rockland Immunochemicals are derived from starting materials of North American origin. Collection was performed in United States Department of Agriculture (USDA) inspected facilities and all materials have been inspected and certified to be free of disease and suitable for exportation. All properties listed are typical characteristics and are not specifications. All suggestions and data are offered in good faith but without guarantee as conditions and methods of use of our products are beyond our control. All claims must be made within 30 days following the date of delivery. The prospective user must determine the suitability of our materials before adopting them on a commercial scale. Suggested uses of our products are not recommendations to use our products in violation of any patent or as a license under any patent of Rockland Immunochemicals, Inc. If you require a commercial license to use this material and do not have one, then return this material, unopened to: Rockland Inc., P.O. BOX 5199, Limerick, Pennsylvania, USA.