Obesity has become a critical global health issue. According to WHO data from 2022, 43% of adults aged 18 and over were overweight, with 16% classified as obese. The prevalence of adult obesity has more than doubled since 1990, and adolescent obesity has quadrupled, affecting countries worldwide regardless of income levels.1 This alarming trend has led to obesity being recognized as a pandemic, although its progression is slower and its impacts more prolonged compared to rapid viral pandemics like COVID-19.2
Obesity and Type 2 Diabetes
Obesity is a significant risk factor for developing type 2 diabetes (T2D) due to the complex interplay between excess body fat and metabolic processes (Reviewed in Klein et al., 2022). The accumulation of excessive fat, particularly in the abdominal region, leads to insulin resistance, a condition where the body's cells become less responsive to insulin. This resistance is exacerbated by the dysfunctional fat cells in obese individuals, which secrete abnormal amounts of adipokines and free fatty acids, contributing to systemic inflammation and further impairing insulin action.
Moreover, obesity-induced insulin resistance involves multiple organs, including the liver, muscles, and pancreatic β-cells. Due to fat accumulation, the liver's increased gluconeogenesis and decreased insulin clearance contribute to higher blood glucose levels. Similarly, muscle cells in obese individuals show impaired glucose uptake and utilization, which is crucial for maintaining normal blood sugar levels.
Pancreatic β-cells initially compensate for insulin resistance by increasing insulin secretion. However, prolonged obesity can lead to β-cell dysfunction and a decrease in insulin production, tipping the balance towards hyperglycemia and the onset of type 2 diabetes. Adipose tissue in obesity undergoes structural changes, such as fibrosis and increased infiltration of inflammatory immune cells, which release cytokines that further disrupt insulin signaling.
Glucagon in Type 2 Diabetes
Glucagon, a hormone produced by differential processing of proglucagon in the α-cells of the pancreas, plays a crucial role in glucose regulation by stimulating hepatic glucose production (Reviewed in Ahrén, 2015). In T2D, glucagon assumes a significant pathological role. Patients with T2D often exhibit elevated baseline glucagon levels that are inadequately suppressed after meals, contributing to persistent hyperglycemia.
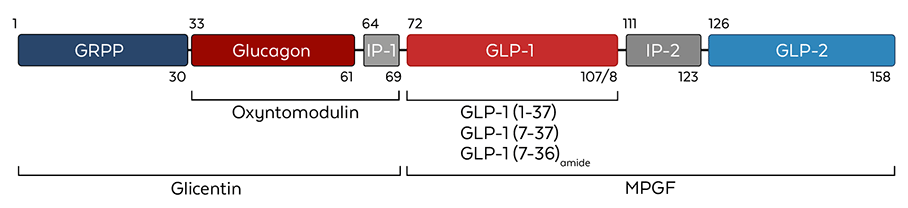 Figure 1. Proglucagon structure and its processed variants. MPGF= major proglucagon fragment. (Adapted from Drucker et al., 2017)
Figure 1. Proglucagon structure and its processed variants. MPGF= major proglucagon fragment. (Adapted from Drucker et al., 2017)
Studies using somatostatin infusion techniques have demonstrated that lowering glucagon levels significantly reduces blood glucose. For instance, in subjects with T2D, reducing glucagon levels during an oral glucose tolerance test diminished the rise in blood glucose, underscoring hyperglucagonemia's substantial contribution to elevated glucose levels in T2D. The mechanism behind this involves impaired regulation of glucagon secretion, possibly due to insulin resistance in alpha cells or defective glucose sensing. This results in increased glucose production by the liver and decreased insulin clearance, both of which are driven by excess glucagon and will ultimately exacerbate hyperglycemia.
Research has shown that therapies targeting glucagon can improve glycemic control in type 2 diabetes. GLP-1 (glucagon-like peptide-1) receptor agonists and DPP-4 (dipeptidyl peptidase-4) inhibitors lower glucagon levels and have proven effective in managing blood sugar levels. These treatments work by enhancing insulin secretion and reducing glucagon secretion, thereby improving overall glucose regulation.
In summary, hyperglucagonemia, driven by the production of glucagon in pancreatic α-cells, is a major factor in the pathology of T2D. Addressing elevated glucagon levels through targeted therapies can significantly enhance glucose control and improve outcomes for individuals with type 2 diabetes.
GLP-1 in Managing Obesity and T2D
GLP-1 (glucagon-like peptide-1) has emerged as a promising focus for obesity and diabetes research. GLP-1 is a peptide hormone produced in the intestinal epithelial endocrine L-cells by differential processing of proglucagon and helps regulate appetite and food intake. It also plays a crucial role in insulin secretion and blood sugar regulation.6 Medications like Ozempic (semaglutide), which are GLP-1 receptor agonists, have shown effectiveness in promoting weight loss and improving blood sugar control in people with type 2 diabetes.7 Understanding and leveraging GLP-1's functions could be a pivotal step in addressing these interconnected health issues.
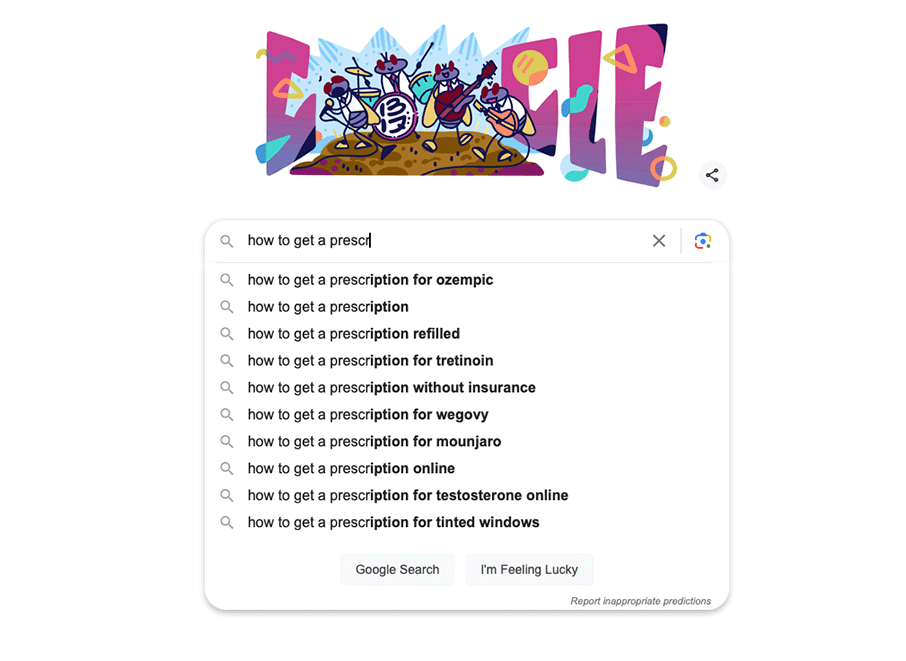 Figure 2. Google autosuggest trends for Ozempic prescription which indicates high volume of searches and a significant public interest for this GLP-1 receptor agonists.
Figure 2. Google autosuggest trends for Ozempic prescription which indicates high volume of searches and a significant public interest for this GLP-1 receptor agonists.