Recombinant VHH Antibodies
Recombinant VHH Antibodies
Realize all the advantages of recombinant VHH antibodies produced by the experienced team at Rockland.
We offer an all-inclusive recombinant VHH antibody production service that generates antibodies suitable for a wide range of applications, including cryptic epitope detection, hapten detection, enzyme active site binding, diagnostic imaging, virus neutralization and more.
Our recombinant VHH antibodies are produced using hyperimmunized camelids and single-cell selection from clonal display, providing the desired sensitivity, specificity, and affinity and enabling production in bacterial or mammalian expression systems.
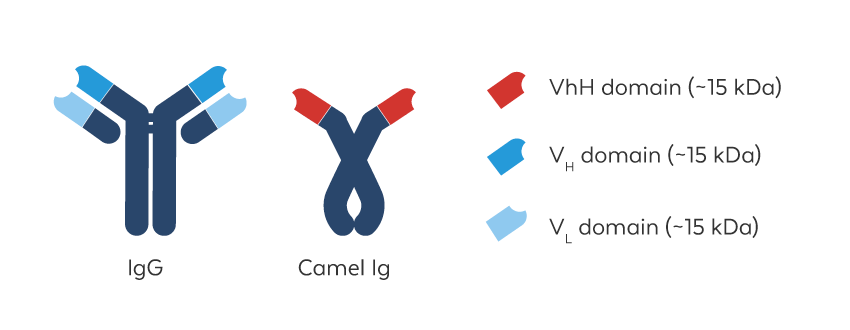
Advantages of VHH Antibodies
Recombinant VHH antibodies, also called nanobodies, are the antigen binding fragment (VHH domain, red) of naturally occurring heavy chain-only antibodies (HCAbs) that are made by hyperimmunized camelids. They can be engineered into monomeric or multimeric, multi-specific formats that possess high target specificity, appropriate affinity, and a low tendency to aggregate. They are also less prone to the steric hindrance problems that can challenge conventional antibodies, making recombinant VHH antibodies ideally suited for a range of pre-clinical diagnostic and therapeutic applications.
Our Recombinant VHH Antibody Production Process
Production of recombinant VHH antibodies is typically an 8-month process that includes antigen preparation, camelid immunization, library construction, biopanning and clone isolation, clone validation, and VHH antibody production.
1
Antigen Preparation
Prior to Month 1
We can use a range of macromolecules as antigens—as long as they can be used under BSL-2 conditions such as protein, recombinant or purified, synthetic peptide, modified peptide, haptens, whole cell lysate, nucleic acid, lipids, and carbohydrate complexes.
2
Camelid Immunization
Month 1–2
We generate VHH antibodies in both llama and alpaca, using the immunization schedule you specify and standard or custom adjuvants. We can provide recommendations prior to library construction based on our decades of experience and monitoring of the antibody-mediated immune response (antisera titration).
3
VHH Library Construction
Month 3
Upon confirmation of a satisfactory immune response, we generate a yeast or phage display library of VHH antibodies. To ensure that the desired binding properties can be found in the library, we analyze critical parameters such as size and diversity.
4
Biopanning & Clone Isolation
Month 3
To isolate clones that bind to the antigen of interest, we use proprietary affinity selection technologies that we’ve developed over decades of successful projects.
5
Recombinant VHH Antibody Validation
Month 4
Isolated single clones are validated using fit-for-purpose studies that include characterization of specificity (binding to the intended target) and cross-reactivity (binding to unrelated antigens) in the conditions relevant to the applications of interest. We can also perform further custom validation for affinity determination, antibody pair identification, and more.
6
Recombinant VHH Antibody Production
Month 5
After validation, clones are expressed in a bacterial system at the desired scale, and then purified at levels suitable for binding and functional studies.
We can also express recombinant VHH antibodies in eukaryotic systems for custom antibody formats and/or applications and use custom signal sequences or N- or C-terminal tags when required.
Interested in VHH antibody production?
Talk with us to find out how we can help move your research forward.