UltraPure Sterile Water
3 References
MB-009-1000
MB-009-4000
1 L
4 x 1 L
Liquid (sterile filtered)
Liquid (sterile filtered)
IHC, LFA
Shipping info:
$50.00 to US & $70.00 to Canada for most products. Final costs are calculated at checkout.
Product Details
UltraPure Sterile Water - MB-009-1000
Deionized water, sterile H2O
Target Details
UltraPure Sterile Water consists of highly polished pharmaceutical grade deionized water processed using sterile conditions. This product was aseptically filtered through a Millipore 0.22 micron filter into clean, pre-sterilized containers. The product was tested on trypticase soy agar for 24 hours, 48 hours and 72 hours and was found to be negative for bacteria.
Application Details
IHC, LFA
- View References
UltraPure Sterile Water is suitable for molecular immunology, molecular biology and nucleic acid methodologies, when applicable.
Formulation
1X
None
None
Shipping & Handling
Ambient
Store container at room temperature (18° to 26° C) prior to opening. If desired, the solution may be stored at 4° C or less.
Expiration date is six (6) months from date of receipt.
Deionized ultrapure water is commonly used in laboratory to prepare buffers in biochemistry and molecular biology laboratories and also cell culture media for cell biology studies.
Sherine F Cheung et al. (2018). A Combined Aqueous Two-Phase System and Spot-Test Platform for the Rapid Detection of Escherichia coli O157:H7 in Milk. SLAS Technol.
Applications
Lateral Flow (LFA)
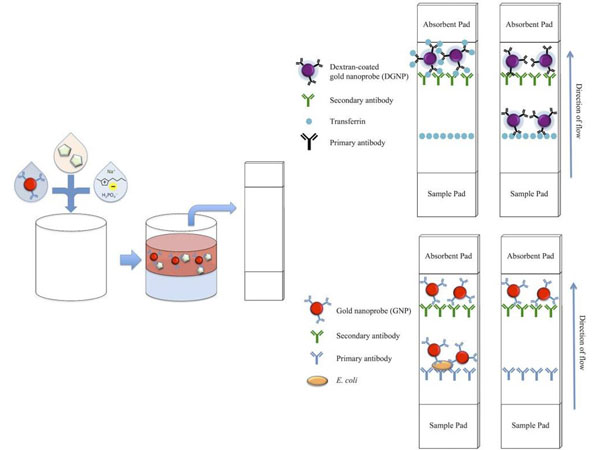
Yee, MF et al. (2018). Ionic Liquid Aqueous Two-Phase Systems for the Enhanced Paper-Based Detection of Transferrin and Escherichia coli. Frontiers in Chemistry
Applications
Buffer
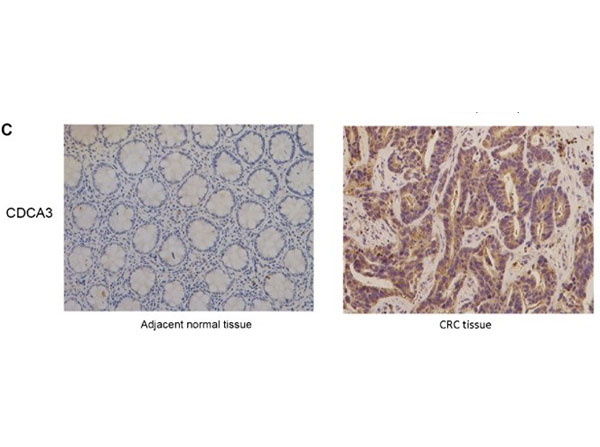
Qian, W et al. (2018). CDCA3 mediates p21-dependent proliferation by regulating E2F1 expression in colorectal cancer. International Journal of Oncology
Applications
IHC, ICC, Histology; Buffer
This product is for research use only and is not intended for therapeutic or diagnostic applications. Please contact a technical service representative for more information. All products of animal origin manufactured by Rockland Immunochemicals are derived from starting materials of North American origin. Collection was performed in United States Department of Agriculture (USDA) inspected facilities and all materials have been inspected and certified to be free of disease and suitable for exportation. All properties listed are typical characteristics and are not specifications. All suggestions and data are offered in good faith but without guarantee as conditions and methods of use of our products are beyond our control. All claims must be made within 30 days following the date of delivery. The prospective user must determine the suitability of our materials before adopting them on a commercial scale. Suggested uses of our products are not recommendations to use our products in violation of any patent or as a license under any patent of Rockland Immunochemicals, Inc. If you require a commercial license to use this material and do not have one, then return this material, unopened to: Rockland Inc., P.O. BOX 5199, Limerick, Pennsylvania, USA.