FACS and IF Staining Buffer with BSA
MB-089-0500
500 mL
Liquid (sterile filtered)
IF, FC
Shipping info:
$50.00 to US & $70.00 to Canada for most products. Final costs are calculated at checkout.
Product Details
Fluorescent Activated Cell Sorting (FACS) and Immunofluorescence (IF) Staining Buffer with BSA - MB-089-0500
Fluorescent Activated Cell Sorting (FACS), Staining buffer, Flow cytometry staining buffer, Immunofluorescence staining buffer
Target Details
FACS and IF Staining Buffer was aseptically filtered through a Millipore 0.22 micron filter into clean, pre-sterilized containers. The product was tested on trypticase soy agar for 24 hours, 48 hours and 72 hours and was found to be negative for bacteria.
Application Details
FC, IF
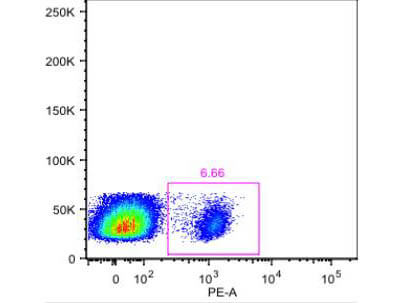
Fluorescent Activated Cell Sorting (FACS) and Immunofluorescence (IF) staining buffer is adequate for cell staining, washing and dilution in flow cytometry and immunofluorescence experiments.
Formulation
1X
0.1% (w/v) Sodium Azide
None
Shipping & Handling
Wet Ice
Store container at 4° C prior to opening.
Expiration date is three (3) months from date of receipt.
Fluorescent Activated Cell Sorting (FACS) and Immunofluorescence (IF) staining buffer is a diluent buffer optimized for use in flow cytometry and immunofluorescence assay of viable and/or fixed cells. FACS and IF staining, washing buffer contains 1% BSA as carrier and stabilizer to reduce non-specific binding of antibodies and fluorochrome reagents to targets cells. Metabolic inhibitors and NaN3 are also included in the flow cytometry and immunofluorescence buffer to prevent patching, capping of surface antigen and bacterial growth.
This product is for research use only and is not intended for therapeutic or diagnostic applications. Please contact a technical service representative for more information. All products of animal origin manufactured by Rockland Immunochemicals are derived from starting materials of North American origin. Collection was performed in United States Department of Agriculture (USDA) inspected facilities and all materials have been inspected and certified to be free of disease and suitable for exportation. All properties listed are typical characteristics and are not specifications. All suggestions and data are offered in good faith but without guarantee as conditions and methods of use of our products are beyond our control. All claims must be made within 30 days following the date of delivery. The prospective user must determine the suitability of our materials before adopting them on a commercial scale. Suggested uses of our products are not recommendations to use our products in violation of any patent or as a license under any patent of Rockland Immunochemicals, Inc. If you require a commercial license to use this material and do not have one, then return this material, unopened to: Rockland Inc., P.O. BOX 5199, Limerick, Pennsylvania, USA.