Datasheet is currently unavailable. Try again or CONTACT US
VlsE Control Protein
3 References
000-001-C33
100 µg
Liquid (sterile filtered)
WB, SDS-PAGE, Biochemical Assay
Borrelia burgdorferi
E. coli
Shipping info:
$50.00 to US & $70.00 to Canada for most products. Final costs are calculated at checkout.
Product Details
VlsE Control Protein - 000-001-C33
Outer surface protein VlsE, Borrelia burgdorferi VlsE, vlsE protein, control protein
Borrelia burgdorferi
E. coli
Recombinant Protein
Target Details
vlsE - View All vlsE Products
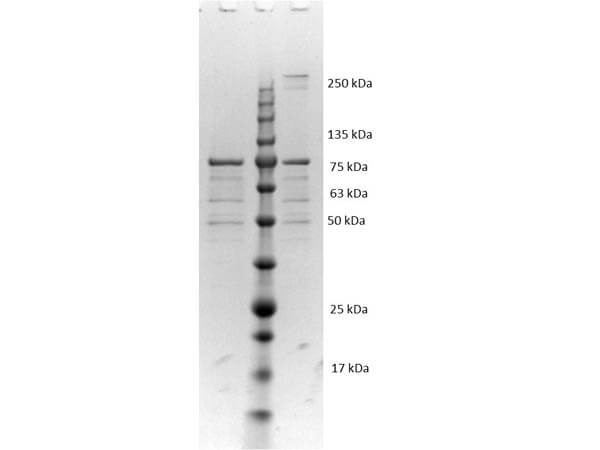
VlsE is a fusion protein with an MBP tag and was expressed in E. coli. Analysis by SDS-PAGE resulted in a pattern consistent with purified VlsE and was estimated to be greater than 90% pure.
Application Details
SDS-PAGE, WB
Biochemical Assay
- View References
VlsE is suitable as a control in immunological assays. Specific conditions for reactivity should be optimized by the end user. Expect a band at 78.7 kDa for VlsE-MBP, (36.3 kDa for VlsE and 42.4 kDa for MBP) in size corresponding to VlsE by Western blotting in the appropriate cell lysate or extract. Variable Lipoprotein Surface-Exposed protein was tested in SDS-page and western blot.
Formulation
1.0 mg/mL by BCA assay
0.02 M Potassium Phosphate, 0.15 M Sodium Chloride, pH 7.2
0.01% (w/v) Sodium Azide
None
Shipping & Handling
Dry Ice
Store vial at -20 °C prior to opening. Aliquot contents and freeze at -20 °C or below for extended storage. Avoid cycles of freezing and thawing. Centrifuge product if not completely clear after standing at room temperature. Dilute only prior to immediate use.
Expiration date is six (6) months from date of receipt.
Variable Lipoprotein Surface-Exposed protein, or VlsE, is a lipoprotein on the surface of the Lyme Disease spirochete Borrelia burgdorferi, detectable during all its life stages. It can exist as many different isoforms. VlsE has variable regions (VRs) and invariable regions (IRs). Some IRs are anchored in the outer membrane of the bacteria and some are antigens exposed on the membrane surface. Replacement of the VR by Borrelia within days of being transferred to a mammalian host presents new surface antigens to the host immune system, and helps Borrelia avoid a strong reaction by host immune systems. The VlsE is apparently not modified as much in the tick or in the rodent vector, when compared to in the mammal host. Several putative envelope proteins of B. burgdorferi appear to be expressed only in the infected mammalian host. The VRs are antigenic, irregularly shaped loops on the bacterial surface which may help to hide both membrane-incorporated and surface portions of adjacent proteins from immune cells. These VR loops are coded by antigenic cassettes. The protein loops can therefore be switched in or out of the protein, or different type loops traded. In B. burgdorferi there seem to be at least fifteen different VlsE cassettes that can insert into any of the variable regions of VlsE, allowing it to appear as millions of different antigens. Similar, but smaller, systems also operate for OSP-A, OSP-B, OSP-C, and other proteins. Some current research involves determination of control of cassette activation. One IR region, C6, of the VlsE protein, consistently stimulates a strong immune response. Its presentation may be a decoy that misdirects the immune system from less protected sites by causing competition for binding antibodies. The bound antibodies are thus not available for binding important therapeutic proteins. This may help Borrelia to enter T-cells, leading to their destruction. Because IR6 is invariable and found in all life stages of B. burgdorferi, it has been used in an ELISA diagnostic test for early IgM of Lyme Disease. Lyme disease proteins are ideal for researchers interested in immunology, neurology, rheumatology, coinfections, autoimmune, and neurodegenerative diseases.
Bowman KA. et al. (2024). Borrelia-specific antibody profiles and complement deposition in joint fluid distinguish antibiotic-refractory from -responsive Lyme arthritis. iScience.
Applications
Protein Assay
Haslund-Gourley BS. et al. (2024). Host glycosylation of immunoglobulins impairs the immune response to acute Lyme disease. eBioMedicine.
Applications
Multiplex Assay
Lone A et al. (2020). The Borrelia burgdorferi VlsE Lipoprotein Prevents Antibody Binding to an Arthritis-Related Surface Antigen. Cell Rep.
Applications
WB, IB, PCA
This product is for research use only and is not intended for therapeutic or diagnostic applications. Please contact a technical service representative for more information. All products of animal origin manufactured by Rockland Immunochemicals are derived from starting materials of North American origin. Collection was performed in United States Department of Agriculture (USDA) inspected facilities and all materials have been inspected and certified to be free of disease and suitable for exportation. All properties listed are typical characteristics and are not specifications. All suggestions and data are offered in good faith but without guarantee as conditions and methods of use of our products are beyond our control. All claims must be made within 30 days following the date of delivery. The prospective user must determine the suitability of our materials before adopting them on a commercial scale. Suggested uses of our products are not recommendations to use our products in violation of any patent or as a license under any patent of Rockland Immunochemicals, Inc. If you require a commercial license to use this material and do not have one, then return this material, unopened to: Rockland Inc., P.O. BOX 5199, Limerick, Pennsylvania, USA.