Datasheet is currently unavailable. Try again or CONTACT US
IL-17F Human Recombinant Protein
009-001-B31
25 µg
Lyophilized
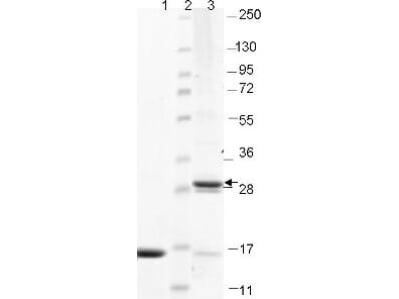
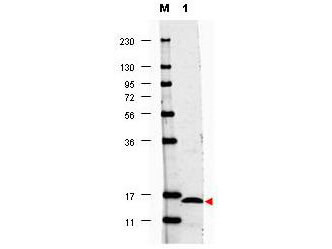
SDS-PAGE
Human
E. coli
Shipping info:
$50.00 to US & $70.00 to Canada for most products. Final costs are calculated at checkout.
Product Details
IL-17F Human Recombinant Protein - 009-001-B31
Interleukin-17F cytokine, IL-17F, Interleukin-24, IL-24, Cytokine ML-1
Human
E. coli
Recombinant Protein
Target Details
IL17F - View All IL17F Products
Purity was determined to be approximately 97% by analysis by RP-HPLC and by reducing and non-reducing SDS-PAGE.
Q96PD4 - UniProtKB
Application Details
SDS-PAGE
IL-17F protein has been tested by SDS-PAGE and is suitable as a control for polyclonal or monoclonal anti-IL-17F in immunological assays.
Formulation
0.1 mg/mL by UV absorbance at 280 nm
0.1% Trifluoroacetic acid
None
None
25µl (25-250µl)
Restore with deionized water (or equivalent)
Shipping & Handling
Ambient
Store vial at 4° C prior to restoration. Dilute only prior to immediate use. Maintain sterility. This product DOES NOT contain preservative. DO NOT VORTEX. We recommend adding a carrier protein such as HSA or BSA to 0.1% (i.e. 1.0 mg/mL) . For best results aliquot contents and freeze at -20° C or colder. Avoid cycles of freezing and thawing. Centrifuge vial before each opening to dislodge contents from the cap and to clarify if contents are not clear after standing at room temperature.
Expiration date is six (6) months from date of receipt.
Recombinant Human Interleukin-17-F is a non-glycosylated protein consisting of two 134 amino acid polypeptide chains that are cysteine linked homodimeric polypeptide chains with a molecular weight of 30 kDa. Homodimeric interleukin-17F is a member of the IL-17 family of six homodimeric proteins (IL-17A-17F). Activity studies reveal IL-17A to be the most potent, followed by IL-17A/F and IL-17F 100 fold lower than IL-17A.
This product is for research use only and is not intended for therapeutic or diagnostic applications. Please contact a technical service representative for more information. All products of animal origin manufactured by Rockland Immunochemicals are derived from starting materials of North American origin. Collection was performed in United States Department of Agriculture (USDA) inspected facilities and all materials have been inspected and certified to be free of disease and suitable for exportation. All properties listed are typical characteristics and are not specifications. All suggestions and data are offered in good faith but without guarantee as conditions and methods of use of our products are beyond our control. All claims must be made within 30 days following the date of delivery. The prospective user must determine the suitability of our materials before adopting them on a commercial scale. Suggested uses of our products are not recommendations to use our products in violation of any patent or as a license under any patent of Rockland Immunochemicals, Inc. If you require a commercial license to use this material and do not have one, then return this material, unopened to: Rockland Inc., P.O. BOX 5199, Limerick, Pennsylvania, USA.