Human IgG
7 References
009-0102-1000
009-0102
1 g
10 mg
Liquid (sterile filtered)
Lyophilized
WB, ELISA, IF, SDS-PAGE, Biochemical Assay
Human
Shipping info:
$50.00 to US & $70.00 to Canada for most products. Final costs are calculated at checkout.
Product Details
Human IgG Whole Molecule - 009-0102
Human IgG whole molecule, Human Immunoglobulin G
Human
IgG
Native Protein
Target Details
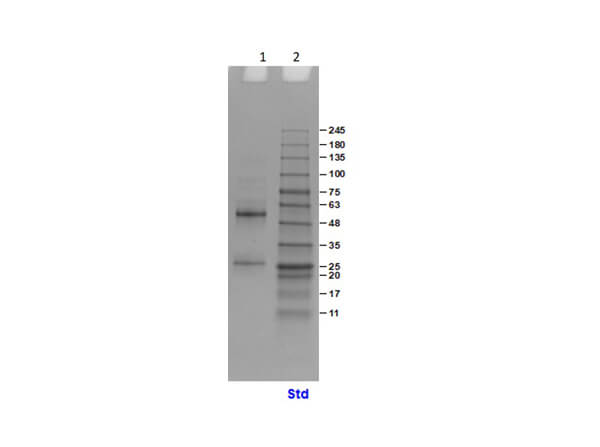
IgG was prepared from normal human serum by a multi-step process which includes delipidation, salt fractionation and ion exchange chromatography followed by extensive dialysis against the buffer stated above. Assay by immunoelectrophoresis resulted in a single precipitin arc against anti-Human IgG and anti-Human Serum.
Application Details
ELISA, SDS-PAGE
Biochemical Assay, IF, WB
- View References
Human IgG whole molecule has been tested by SDS-Page and ELISA and can be utilized as a control or standard reagent in Western Blotting, Flow, and ELISA experiments.
Formulation
10.846 mg/mL by UV absorbance at 280 nm
0.02 M Potassium Phosphate, 0.15 M Sodium Chloride, pH 7.2
0.01% (w/v) Sodium Azide
None
1.0 mL
Restore with deionized water (or equivalent)
Shipping & Handling
Ambient
Store Human IgG at 4° C prior to restoration. For extended storage aliquot contents and freeze at -20° C or below. Avoid cycles of freezing and thawing. Centrifuge product if not completely clear after standing at room temperature. This product is stable for several weeks at 4° C as an undiluted liquid. Dilute only prior to immediate use.
Expiration date is one (1) year from date of receipt.
Human IgG purified protein (Immunoglobulin G) are antibody molecules. Human IgG is composed of four peptide chains — two heavy chains gamma and two light chains. Human IgG has two antigen binding sites. Other Immunoglobulins may be described in terms of polymers with the IgG structure considered the monomer. Human IgG typically constitutes 75% of serum immunoglobulins. Human IgG molecules are synthesized and secreted by plasma B cells.
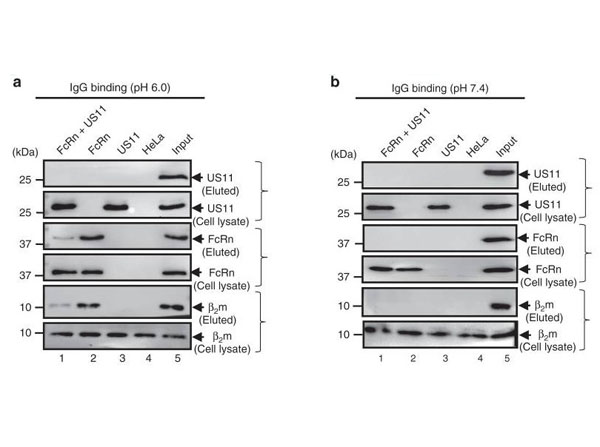
Oh W et al. (2025). Lactic acid inhibits the interaction between PD-L1 protein and PD-L1 antibody in the PD-1/PD-L1 blockade therapy-resistant tumor. Mol Ther.
Applications
EIA, ELISA; Receptor Binding Assays
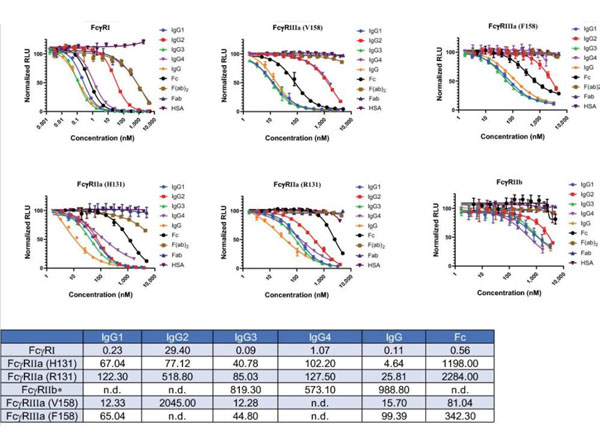
Nath, N et al. (2022). A homogeneous bioluminescent immunoassay for parallel characterization of binding between a panel of antibodies and a family of Fcγ receptors. Scientific Reports
Applications
Receptor Binding Assays
Stoltenburg R et al. (2018). Refining the Results of a Classical SELEX Experiment by Expanding the Sequence Data Set of an Aptamer Pool Selected for Protein A. Int J Mol Sci.
Applications
SPR-Based Analyses
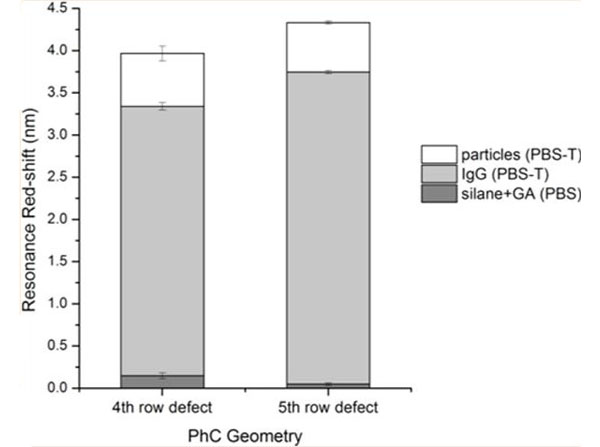
Baker JE et al. (2017). Recognition-mediated particle detection under microfluidic flow with waveguide-coupled 2D photonic crystals: towards integrated photonic virus detectors. Lab Chip.
Applications
Other
Wang, H et al. (2015). Small-Animal PET Imaging of Pancreatic Cancer Xenografts Using a 64Cu-Labeled Monoclonal Antibody, MAb159. Journal of Nuclear Medicine : Official Publication, Society of Nuclear Medicine
Applications
Bead Conjugation
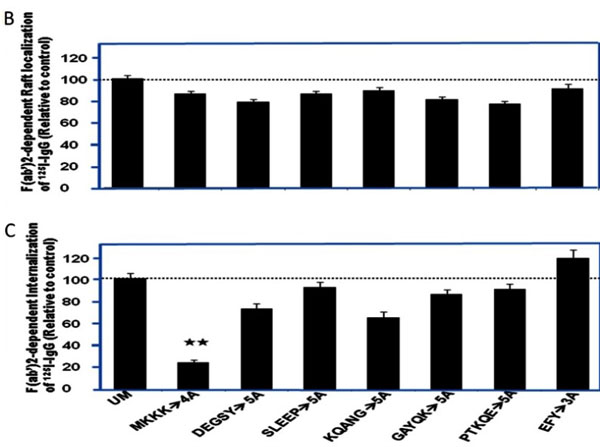
Chen K et al. (2013). Molecular mediators for raft-dependent endocytosis of syndecan-1, a highly conserved, multifunctional receptor. J Biol Chem.
Applications
WB, IB, PCA
Li, D et al. (2013). Targeting the EphB4 receptor for cancer diagnosis and therapy monitoring. Molecular Pharmaceutics
Applications
Receptor Binding Assays; IF, Confocal Microscopy
No test method can provide total assurance that the hepatitis B virus, hepatitis C virus, human immunodeficiency virus, or any other infectious agents are absent. Thus, all blood products, including purified proteins derived from human blood sources, should be handled at Biosafety Level 2 as recommended by the CDC/NIH manual entitled Biosafety in Microbiological and Biomedical Laboratories for potentially infectious human serum, blood specimens or proteins derived from same. Source material for the human blood product supplied to your facility has been tested for the detection of HIV antibody, Hepatitis B surface antigen, antibody to Hepatitis C, HIV 1 antigen(s), antibody to HTLV - I/II, and syphilis by FDA guidelines. All units were found to be non-reactive/negative for these tests. All human blood source material is collected in FDA licensed centers and is tested with FDA approved test kits.; This product is for research use only and is not intended for therapeutic or diagnostic applications. Please contact a technical service representative for more information. All products of animal origin manufactured by Rockland Immunochemicals are derived from starting materials of North American origin. Collection was performed in United States Department of Agriculture (USDA) inspected facilities and all materials have been inspected and certified to be free of disease and suitable for exportation. All properties listed are typical characteristics and are not specifications. All suggestions and data are offered in good faith but without guarantee as conditions and methods of use of our products are beyond our control. All claims must be made within 30 days following the date of delivery. The prospective user must determine the suitability of our materials before adopting them on a commercial scale. Suggested uses of our products are not recommendations to use our products in violation of any patent or as a license under any patent of Rockland Immunochemicals, Inc. If you require a commercial license to use this material and do not have one, then return this material, unopened to: Rockland Inc., P.O. BOX 5199, Limerick, Pennsylvania, USA.