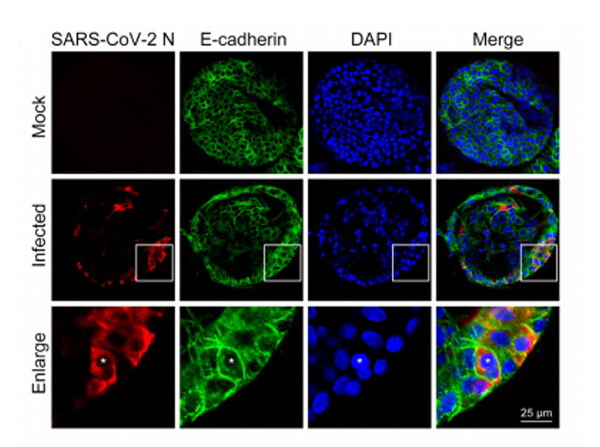
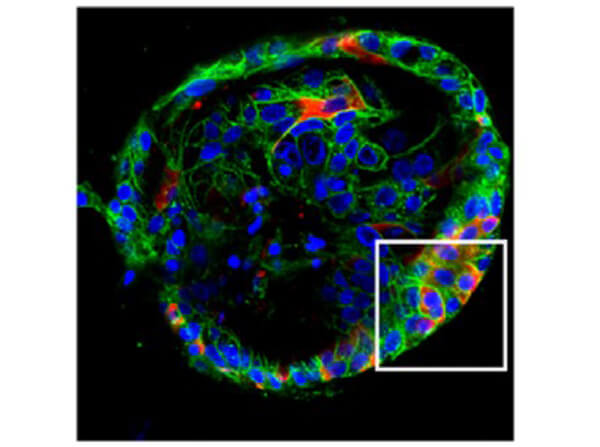
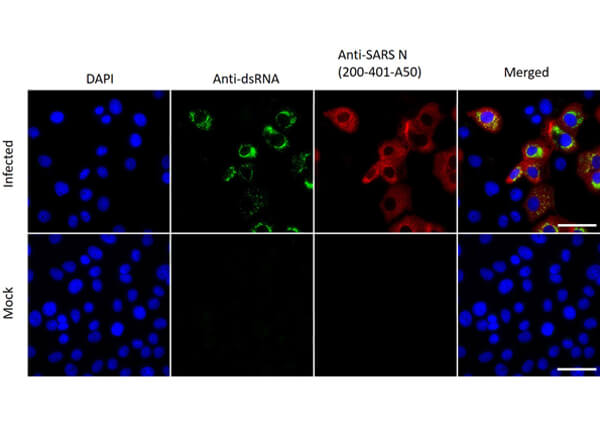
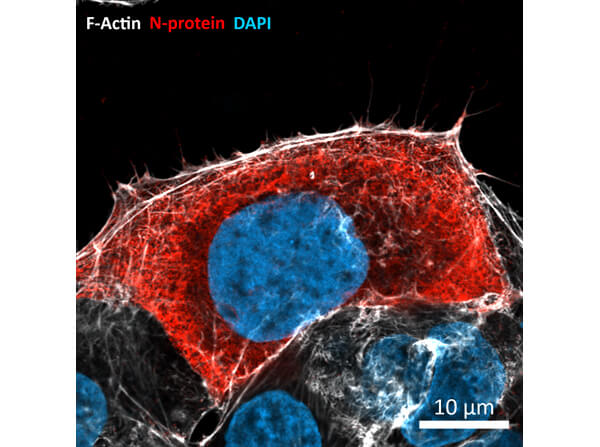
Sars Nucleocapsid Protein Antibody
Rabbit Polyclonal
105 References
200-401-A50
500 µg
Lyophilized
WB, ELISA, IHC, IF, FC, FISH, LFA, Multiplex, Other
Virus
Rabbit
Shipping info:
$50.00 to US & $70.00 to Canada for most products. Final costs are calculated at checkout.
Product Details
Anti-SARS-CoV Nucleocapsid (N) Protein (RABBIT) Antibody - 200-401-A50
rabbit anti-Sars Nucleocapsid Protein Antibody, rabbit anti-Sars-CoV Nucleocapsid (N) Protein Antibody, N antibody, N structural protein antibody, NC antibody, Nucleocapsid protein antibody, Nucleoprotein antibody, SARS coronavirus N protein antibody, SARS CoV antibody, SARSCoV antibody, Severe acute respiratory syndrome antibody, A50 Antibody, A50 SARS, COVID
Rabbit
Polyclonal
IgG
Target Details
Virus
Recombinant Protein
This protein A purified antibody was prepared from whole rabbit serum produced by repeated immunizations with a purified recombinant protein corresponding to full length SARS Coronavirus Nucleocapsid protein. Lifesensors Inc. (www.lifesensors.com) prepared the Nucleocapsid protein as follows: SUMO-Nucleocapsid fusion was expressed in E.coli in LB medium and purified using Ni-NTA resin (Qiagen) affinity chromatography. After the fusion was cleaved by the SUMO Protease (LifeSensors), the SUMO tag and protease were subtracted from the nucleocapsid using MAC and the nucleocapsid was finally purified using Cation Exchange Chromatography with the Macro-Prep High S resin (BioRad) and size exclusion chromatography.
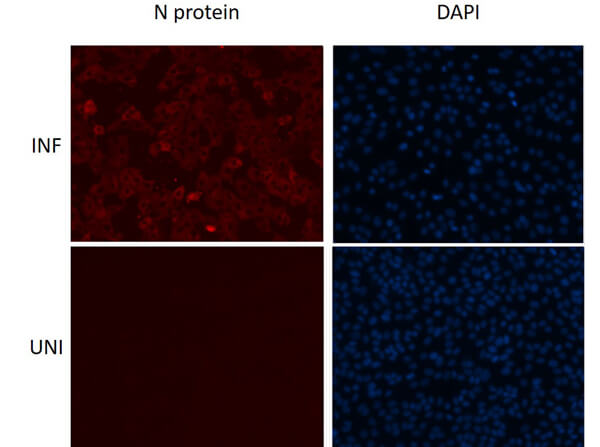
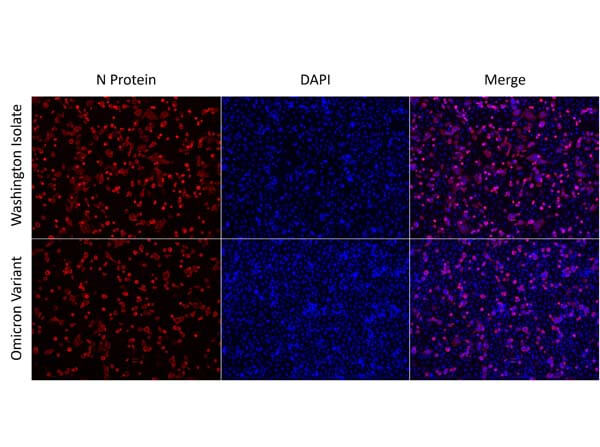
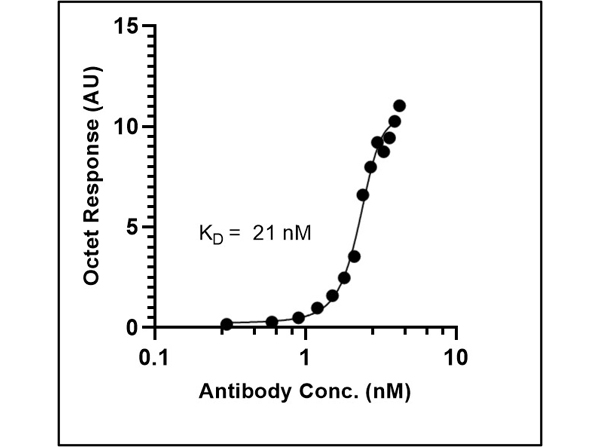
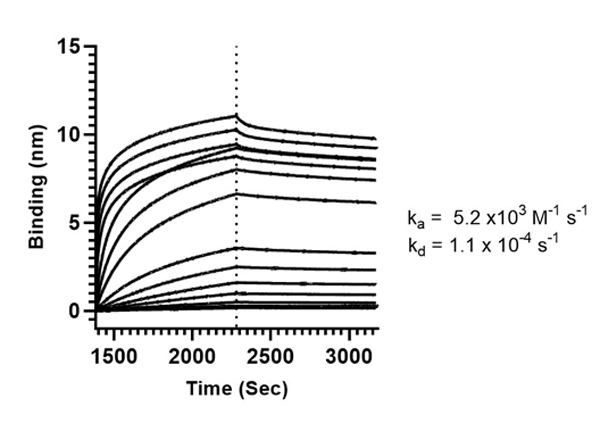
This protein A purified antibody is directed against SARS Coronavirus Nucleocapsid (N) protein. The product was purified from monospecific antiserum by protein A affinity purification. Detects the Nucleocapsid (N), Omicron, and BA.2 sub-variant. BLAST analysis was used to suggest reactivity with related Coronavirus proteins. Cross reactivity with homologues from other sources has not been determined.
Application Details
ELISA, WB
FC, FISH, IF, IHC, Other, LFA, Multiplex
- View References
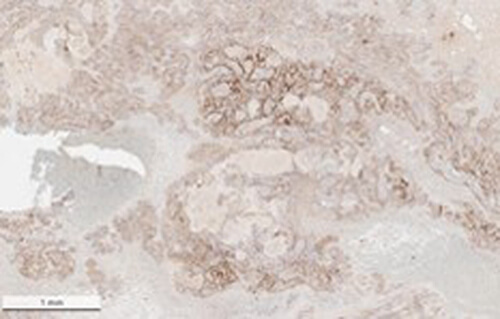
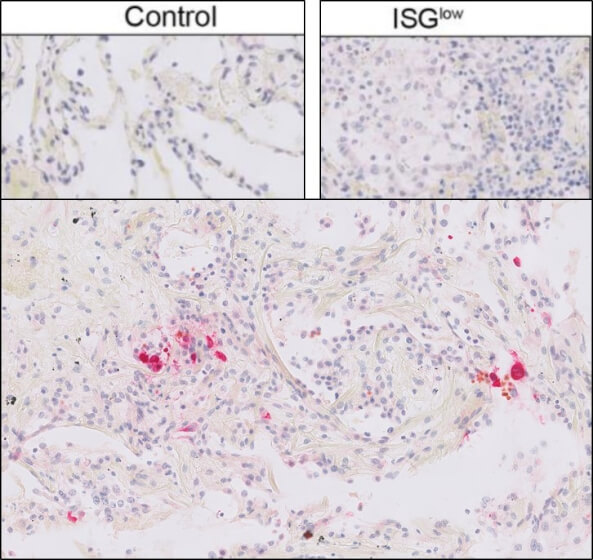
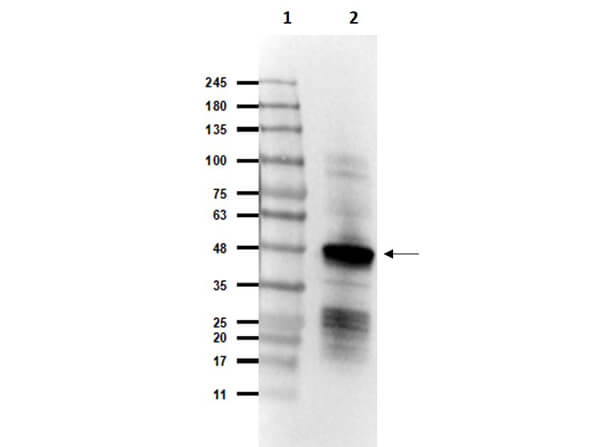
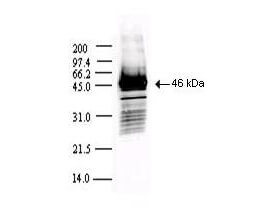
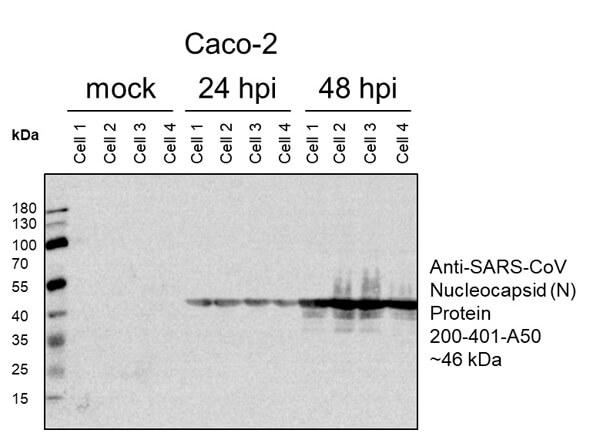
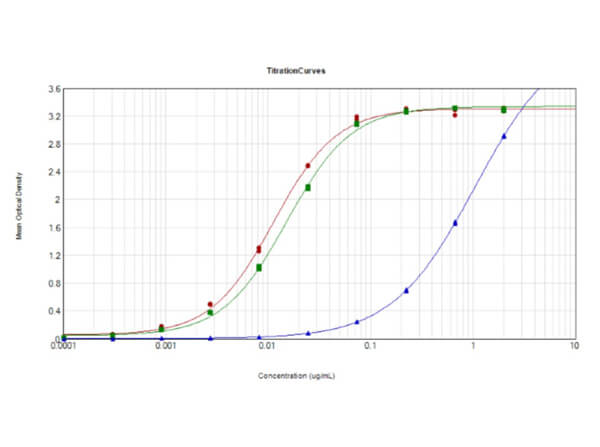
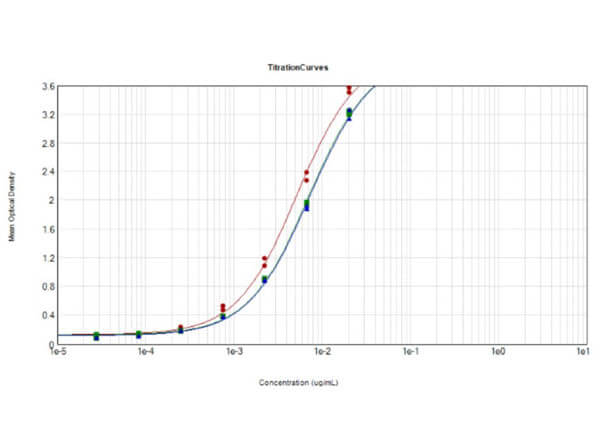
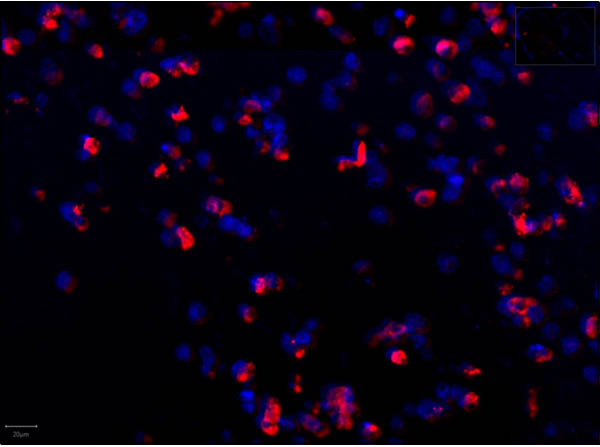
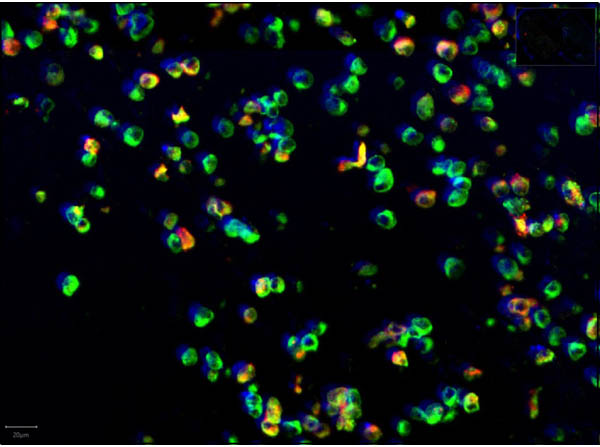
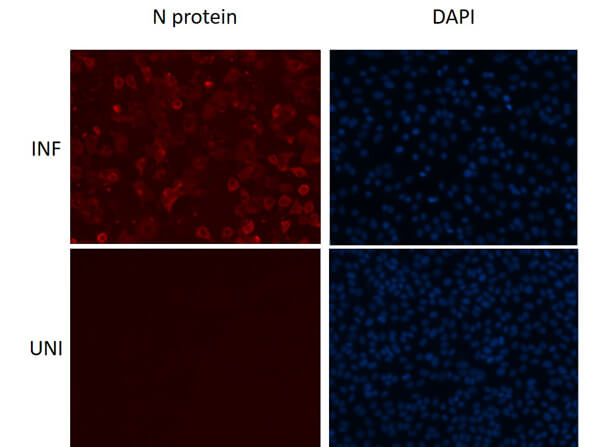
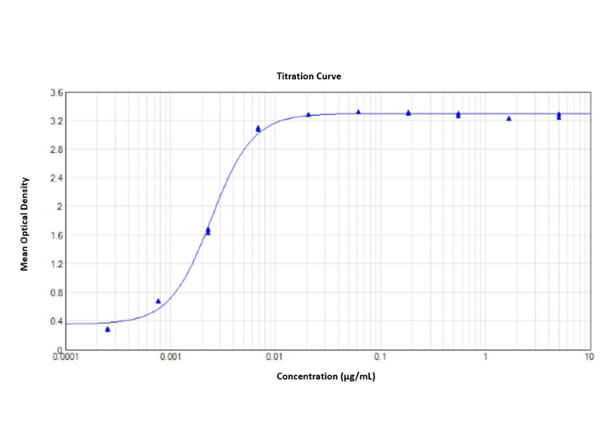
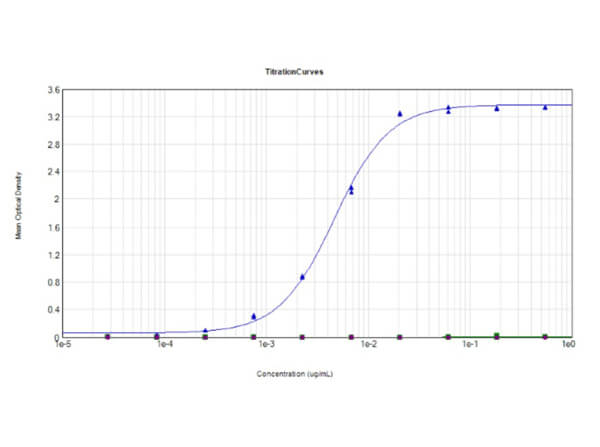
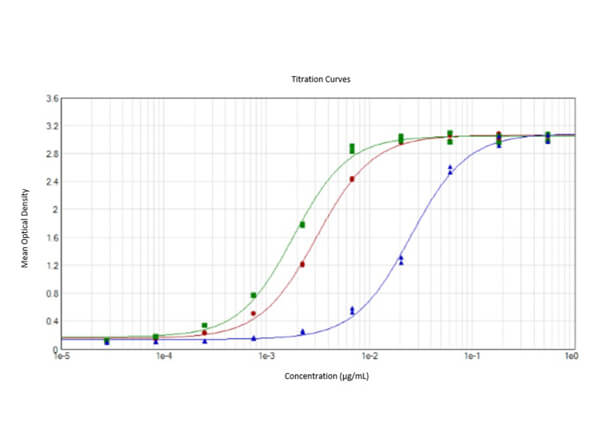
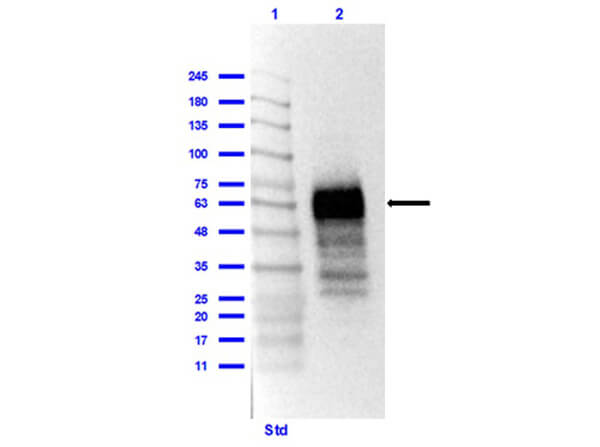
This protein A purified antibody has been tested for use in ELISA, western blot, Immunohistochemistry, Immunofluorescence, and lateral flow. Specific conditions for reactivity should be optimized by the end user. Expect a band approximately 46 kDa in size corresponding to SARS Nucleocapsid (N) protein by western blotting in the appropriate cell lysate or extract. ELISA and lateral flow format has been used to detect virus in extracts from nasal and throat swabs and saliva. IF has been used to determine the presence or absence of virus entering cells especially when anti-viral drugs are applied. IHC studies have been performed on biopsies, included retrospective studies on cadaver tissues after formalin fixation and paraffin embedding, detecting the coronavirus in lung, liver, bile duct, and placenta tissue. Yet other studies have shown this antibody has the ability to neutralize the virus and thereby protect cells from the uptake of live virus. Others have demonstrated the utility of the antibody in flow cytometry studies.
Formulation
5.3 mg/mL by UV absorbance at 280 nm
0.02 M Potassium Phosphate, 0.15 M Sodium Chloride, pH 7.2
0.01% (w/v) Sodium Azide
None
100 µL
Restore with deionized water (or equivalent)
Shipping & Handling
Ambient
Store vial at 4° C prior to restoration. For extended storage aliquot contents and freeze at -20° C or below. Avoid cycles of freezing and thawing. Centrifuge product if not completely clear after standing at room temperature. This product is stable for several weeks at 4° C as an undiluted liquid. Dilute only prior to immediate use.
Expiration date is one (1) year from date of receipt.
The coronavirus nucleocapsid protein is the major structural component of virions that associates with genomic RNA to form a long, flexible, helical nucleocapsid. Sequence comparison of the N genes of five strains of the coronavirus mouse hepatitis virus suggests a three-domain structure for the nucleocapsid protein. Anti-SARS-CoV Nucleocapsid (N) Protein Antibody is useful for researchers interested in viral research.
Barlang LA et al. (2024). Distribution and suitability of pulmonary surfactants as a vehicle for topically applied antibodies in healthy and SARS-CoV-2 infected rodent lungs. Eur J Pharm Sci.
Applications
IHC, ICC, Histology
Du L et al. (2024). A viral assembly inhibitor blocks SARS-CoV-2 replication in airway epithelial cells. Commun Biol.
Applications
WB, IB, PCA
Brogna C et al. (2024). Who Is the Intermediate Host of RNA Viruses? A Study Focusing on SARS-CoV-2 and Poliovirus. Microorganisms.
Applications
IF, Confocal Microscopy
Weingarten-Gabbay S et al. (2024). The HLA-II immunopeptidome of SARS-CoV-2. Cell Rep.
Applications
IF, Confocal Microscopy
Gallucci L et al. (2024). Broad-spectrum antiviral activity of two structurally analogous CYP3A inhibitors against pathogenic human coronaviruses in vitro. Antiviral Res.
Applications
IF, Confocal Microscopy
Bagato O et al. (2024). Spatiotemporal analysis of SARS-CoV-2 infection reveals an expansive wave of monocyte-derived macrophages associated with vascular damage and virus clearance in hamster lungs. Microbiol Spectr.
Applications
IHC, ICC, Histology
Vanderlinden E et al. (2023). PRO-2000 exhibits SARS-CoV-2 antiviral activity by interfering with spike-heparin binding. Antiviral Res.
Applications
Viral Assay
Stüdle C et al. (2023). SARS-CoV-2 infects epithelial cells of the blood-cerebrospinal fluid barrier rather than endothelial cells or pericytes of the blood-brain barrier. Fluids Barriers CNS.
Applications
IF, Confocal Microscopy
Chen DY et al. (2023). Spike and nsp6 are key determinants of SARS-CoV-2 Omicron BA.1 attenuation. Nature.
Applications
IF, Confocal Microscopy; WB, IB, PCA
Li L et al. (2023). Plant flavonoid inhibition of SARS-CoV-2 main protease and viral replication. iScience.
Applications
IF, Confocal Microscopy
Brogna C et al. (2023). Analysis of Bacteriophage Behavior of a Human RNA Virus, SARS-CoV-2, through the Integrated Approach of Immunofluorescence Microscopy, Proteomics and D-Amino Acid Quantification. Int J Mol Sci.
Applications
IF, Confocal Microscopy
Huang X et al. (2023). SARS-CoV-2 induces cardiomyocyte apoptosis and inflammation but can be ameliorated by ACE inhibitor Captopril. Antiviral Res.
Applications
IF, Confocal Microscopy
Fraternale A et al. (2023). Targeting SARS-CoV-2 by synthetic dual-acting thiol compounds that inhibit Spike/ACE2 interaction and viral protein production. FASEB J.
Applications
WB, IB, PCA
Mäkelä AR et al. (2023). Intranasal trimeric sherpabody inhibits SARS-CoV-2 including recent immunoevasive Omicron subvariants. Nat Commun.
Applications
Neutralization
Chen DY et al. (2023). Cell culture systems for isolation of SARS-CoV-2 clinical isolates and generation of recombinant virus. iScience.
Applications
IF, Confocal Microscopy
iScience. (2023). F-actin nanostructures rearrangements and regulation are essential for SARS-CoV-2 particle production in host pulmonary cells. Swain J et al.
Applications
Undefined
Gao S et al. (2023). Endothelial SARS-CoV-2 infection is not the underlying cause of COVID-19-associated vascular pathology in mice. Front Cardiovasc Med.
Applications
IF, Confocal Microscopy
Buzas D et al. (2023). In vitro generated antibodies guide thermostable ADDomer nanoparticle design for nasal vaccination and passive immunization against SARS-CoV-2. Antib Ther.
Applications
Neutralization
Tang AT et al. (2023). Cell-autonomous requirement for ACE2 across organs in lethal mouse SARS-CoV-2 infection. PLoS Biol.
Applications
IHC, ICC, Histology
Koutsakos M et al. (2023). SARS-CoV-2 breakthrough infection induces rapid memory and de novo T cell responses. Immunity.
Applications
EIA, ELISA
Datta S et al. (2023). High-resolution photocatalytic mapping of SARS-CoV-2 spike interactions on the cell surface. Cell Chem Biol.
Applications
Undefined
Torchia JA et al. (2022). Optimized ACE2 decoys neutralize antibody-resistant SARS-CoV-2 variants through functional receptor mimicry and treat infection in vivo. Sci Adv.
Applications
FC, FACS, FLOW
Halliday A et al. (2022). Development and evaluation of low-volume tests to detect and characterize antibodies to SARS-CoV-2. Front Immunol.
Applications
Microneutralization Assay (MNT)
Ren W et al. (2022). Susceptibilities of Human ACE2 Genetic Variants in Coronavirus Infection. J Virol.
Applications
IF, Confocal Microscopy
Barut GT et al. (2022). The spike gene is a major determinant for the SARS-CoV-2 Omicron-BA.1 phenotype. Nat Commun.
Applications
IHC, ICC, Histology
Beer J et al. (2022). Impaired immune response drives age-dependent severity of COVID-19. J Exp Med.
Applications
IHC, ICC, Histology
Gourdelier M et al. (2022). Optimized production and fluorescent labeling of SARS-CoV-2 virus-like particles. Sci Rep.
Applications
WB, IB, PCA
Seliger B et al. (2022). Induction of pulmonary HLA-G expression by SARS-CoV-2 infection. Cell Mol Life Sci.
Applications
IHC, ICC, Histology
Rocha SM. et al. (2022). A Novel Glucocorticoid and Androgen Receptor Modulator Reduces Viral Entry and Innate Immune Inflammatory Responses in the Syrian Hamster Model of SARS-CoV-2 Infection. Front Immunol.
Applications
IHC, ICC, Histology
Wang R et al. (2022). Human airway lineages derived from pluripotent stem cells reveal the epithelial responses to SARS-CoV-2 infection. Am J Physiol Lung Cell Mol Physiol.
Applications
IF, Confocal Microscopy
Taglauer ES. et al. (2022). Acute Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Pregnancy Is Associated with Placental Angiotensin-Converting Enzyme 2 Shedding. Am J Pathol.
Applications
IHC, ICC, Histology
Kaleta T et al. (2022). Antibody escape and global spread of SARS-CoV-2 lineage A. 27. Nat Commun.
Applications
IF, Confocal Microscopy
Brogna C et al. (2022). Could SARS-CoV-2 Have Bacteriophage Behavior or Induce the Activity of Other Bacteriophages? Vaccines (Basel).
Applications
IF, Confocal Microscopy
Simonetti B et al. (2022). ESCPE-1 Mediates Retrograde Endosomal Sorting of the SARS-CoV-2 Host Factor Neuropilin-1. Proc Natl Acad Sci USA
Applications
IF, Confocal Microscopy
Wragg KM et al. (2022). Establishment and recall of SARS-CoV-2 spike epitope-specific CD4+ T cell memory. Nat Immunol.
Applications
EIA, ELISA
Chen M et al. (2022). Evolution of nasal and olfactory infection characteristics of SARS-CoV-2 variants. bioRxiv Preprint
Applications
IHC, ICC, Histology
Murer L et al. (2022). Identification of broad anti-coronavirus chemical agents for repurposing against SARS-CoV-2 and variants of concern. Curr Res Virol Sci.
Applications
EIA, ELISA
Jang D et al. (2022). Low molecular weight chitooligosaccharide inhibits infection of SARS‐CoV‐2 in vitro. J Appl Microbiol.
Applications
IF, Confocal Microscopy
Gupta K et al. (2022). Structural insights in cell-type specific evolution of intra-host diversity by SARS-CoV-2. Nat Commun.
Applications
Cell Proliferation Assay
Koutsakos M et al. (2022). The magnitude and timing of recalled immunity after breakthrough infection is shaped by SARS-CoV-2 variants. Immunity.
Applications
EIA, ELISA
Kannan, A et sl. (2022). Sequence-Specific Features of Short Double-Strand, Blunt-End RNAs Have RIG-I- and Type 1 Interferon-Dependent or -Independent Anti-Viral Effects. Viruses
Applications
IF, Confocal Microscopy
Taddeo, A et al. (2022). Optimized intramuscular immunization with VSV-vectored spike protein triggers a superior immune response to SARS-CoV-2. Npj Vaccines
Applications
IHC, ICC, Histology
Ju Y et al. (2022). Anti-PEG Antibodies Boosted in Humans by SARS-CoV-2 Lipid Nanoparticle mRNA Vaccine. Acs Nano
Applications
Neutralization
Shi FS et al. (2022). Expression Profile and Localization of SARS-CoV-2 Nonstructural Replicase Proteins in Infected Cells. Microbiol Spectr.
Applications
IF, Confocal Microscopy
de Vries M et al. (2021). A comparative analysis of SARS-CoV-2 antivirals characterizes 3CL pro inhibitor PF-00835231 as a potential new treatment for COVID-19. J Virol.
Applications
IF, Confocal Microscopy
Haslbauer JD. et al. (2021). Histomorphological patterns of regional lymph nodes in COVID-19 lungs. Pathologist
Applications
IHC, ICC, Histology
Zhu Y. et al. (2021). A genome-wide CRISPR screen identifies host factors that regulate SARS-CoV-2 entry. Nat Commun.
Applications
IHC, ICC, Histology
Williamson MK et al. (2021). Chronic SARS-CoV-2 infection and viral evolution in a hypogammaglobulinaemic individual. medRxiv Preprint
Applications
IF, Confocal Microscopy
Goenka A et al. (2021). Young infants exhibit robust functional antibody responses and restrained IFN-γ production to SARS-CoV-2. Cell Rep Med.
Applications
IF, Confocal Microscopy; NEU
Weingarten-Gabby S et al. (2021). Profiling SARS-CoV-2 HLA-I peptidome reveals T cell epitopes from out-of-frame ORFs. Cell.
Applications
IF, Confocal Microscopy
Lopez E et al. (2021). Simultaneous evaluation of antibodies that inhibit SARS-CoV-2 variants via multiplex assay. JCI Insight.
Applications
E, EIA; NEU
Sanders DW et al. (2021). SARS-CoV-2 requires cholesterol for viral entry and pathological syncytia formation. Elife.
Applications
IF, Confocal Microscopy
Thepaut M et al. (2021). DC/L-SIGN recognition of spike glycoprotein promotes SARS-CoV-2 trans-infection and can be inhibited by a glycomimetic antagonist. PLoS Pathog.
Applications
E, EIA
Steinlin-Schopfer J et al. (2021). Evaluation of the Roche antigen rapid test and a cell culture-based assay compared to rRT- PCR for the detection of SARS-CoV-2: A contribution to the discussion about SARS-CoV-2 diagnostic tests and contagiousness. Journal of Clinical Virology Plus Preprint
Applications
IF, Confocal Microscopy
Gupta K et al. (2021). Structure and mechanism of SARS-CoV-2 Spike N679-V687 deletion variant elucidate cell-type specific evolution of viral fitness. bioRxiv Preprint
Applications
IF, Confocal Microscopy
Klouda T et al. (2021). Interferon-alpha or-beta facilitates SARS-CoV-2 pulmonary vascular infection by inducing ACE2. Angiogenesis
Applications
IHC, ICC, Histology
Avolio E et al. (2021). The SARS-CoV-2 Spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: a potential non-infective mechanism of COVID-19 microvascular disease. Clin Sci (Lond).
Applications
IF, Confocal Microscopy
Pfafenrot C. et al. (2021). Inhibition of SARS-CoV-2 coronavirus proliferation by designer antisense-circRNAs. Nuckeic Acids Res.
Applications
WB, IB, PCA
Wheatley AK. et al. (2021). Landscape of human antibody recognition of the SARS-CoV-2 receptor binding domain. Cell Rep.
Applications
EIA, ELISA
Taglauer ES. et al. (2021). Acute SARS-CoV-2 infection in pregnancy is associated with placental ACE-2 shedding. bioRxiv Preprint
Applications
IHC, ICC, Histology
De Angelis M et al. (2021). Protective Role of Combined Polyphenols and Micronutrients against Influenza A Virus and SARS-CoV-2 Infection In Vitro. Biomedicines
Applications
WB, IB, PCA
De Santis R et al. (2021). Rapid inactivation of SARS-CoV-2 with LED irradiation of visible spectrum wavelengths. J Photochem Photobiol.
Applications
WB, IB, PCA
Weigang S et al. (2021). Within-host evolution of SARS-CoV-2 in an immunosuppressed COVID-19 patient: a source of immune escape variants. Nat Commun.
Applications
IF, Confocal Microscopy; WB, IB, PCA
Sadeghi P et al. (2021). Lateral flow assays (LFA) as an alternative medical diagnosis method for detection of virus species: The intertwine of nanotechnology with sensing strategies. Trends Analyt Chem.
Applications
Lateral Flow (LFA)
Matusali G et al. (2021). Pleural Mesothelial Cells Modulate the Inflammatory/Profibrotic Response During SARS-CoV-2 Infection. Front Mol Biosci.
Applications
IF, Confocal Microscopy
Koutsakos M et al. (2021). Dynamics of immune recall following SARS-CoV-2 vaccination or breakthrough infection. medRxiv Preprint
Applications
EIA, ELISA
Juno J et al. (2021). Tracking the kinetics and phenotype of spike epitope-specific CD4 T cell immunity in the context of SARS-CoV-2 infection and vaccination. Research Square Preprint
Applications
IHC, ICC, Histology
Tang AT et al. (2021). SARS-CoV-2 infection of olfactory epithelial cells and neurons drives acute lung injury and lethal COVID-19 in mice bioRxiv Preprint
Applications
IHC, ICC, Histology
Ching KL et al. (2021). ACE2-containing defensosomes serve as decoys to inhibit SARS-CoV-2 infection. bioRxiv Preprint
Applications
IF, Confocal Microscopy
Godbole I et al. (2021). Engineered chimeric T cell receptor fusion construct (TRuC)-expressing T cells prevent translational shutdown in SARS-CoV-2-infected cells. bioRxiv Preprint
Applications
IF, Confocal Microscopy
Murer LP et al. (2021). Arrayed multicycle drug screens identify broadly acting chemical inhibitors for repurposing against SARS CoV 2. bioRxiv Preprint
Applications
IF, Confocal Microscopy
Haslbauer JD. et al. (2021). Characterisation of cardiac pathology in 23 autopsies of lethal COVID‐19. J Pathol Clin Res.
Applications
IHC, ICC, Histology
Merdji H et al. (2021). Histopathological features in fatal COVID-19 acute respiratory distress syndrome. Med Intensiva.
Applications
ISH, FISH
Ciccosanti F et al. (2021). Proteomic Analysis Identifies the RNA Helicase DDX3X as a Host Target Against SARS-CoV-2 Infection. Antiviral Res.
Applications
WB, IB, PCA
Jung S et al. (2021). Copper-Coated Polypropylene Filter Face Mask with SARS-CoV-2 Antiviral Ability. Polymers (Basel).
Applications
IF, Confocal Microscopy
Chen DY et al. (2021). SARS-CoV-2 disrupts proximal elements in the JAK-STAT pathway. J Virol.
Applications
IF, Confocal Microscopy
Gultom M. et al. (2021). Susceptibility of well-differentiated airway epithelial cell cultures from domestic and wildlife animals to SARS-CoV-2. Emerg Infect Dis.
Applications
IF, Confocal Microscopy
Shaban MS. et al. (2021). Multi-level inhibition of coronavirus replication by chemical ER stress. Nat Commun.
Applications
WB, IB, PCA; E, EIA
de Vries M et al. (2021). Comparative study of a 3CLpro inhibitor and remdesivir against both major SARS-CoV-2 clades in human airway models. bioRxiv Preprint
Applications
IF, Confocal Microscopy
Merdji, H et al. (2021). Histopathological features in fatal COVID-19 acute respiratory distress syndrome. Medicina Intensiva
Applications
IHC, ICC, Histology
Haslbauer, JD et al. (2021). Histomorphological patterns of regional lymph nodes in COVID-19 lungs. Der Pathologe
Applications
IHC, ICC, Histology
Cate D et al. (2020). Antibody Screening Results for Anti-Nucleocapsid Antibodies Towards the Development of a SARS-CoV-2 Nucleocapsid Protein Antigen Detecting Lateral Flow Assay chemRxiv Preprint
Applications
Lateral Flow (LFA)
Zhao B. et al. (2020). Recapitulation of SARS-CoV-2 Infection and Cholangiocyte Damage with Human Liver Organoids. Protein Cell
Applications
IF, Confocal Microscopy; Multiplex Assay
Thao T. et al. (2020). Rapid reconstruction of SARS-CoV-2 using a synthetic genomics platform. Nature
Applications
IF, Confocal Microscopy
Holwerda M. et al. (2020). Identification of an Antiviral Compound from the Pandemic Response Box that Efficiently Inhibits SARS-CoV-2 Infection In Vitro. Microorganisms
Applications
IF, Confocal Microscopy
Duan X. et al. (2020). Identification of Drugs Blocking SARS-CoV-2 Infection using Human Pluripotent Stem Cell-derived Colonic Organoids. bioRxiv Preprint
Applications
IF, Confocal Microscopy
Bouhaddou M, Memon D, Meyer B, et al. (2020). The Global Phosphorylation Landscape of SARS-CoV-2 Infection. Cell
Applications
IF, Confocal Microscopy; Multiplex Assay
Loffredo M. et al. (2020). The in-vitro effect of famotidine on sars-cov-2 proteases and virus replication. Sci Rep.
Applications
IF, Confocal Microscopy
Yurkovetskiy L. et al. (2020). Structural and Functional Analysis of the D614G SARS-CoV-2 Spike Protein Variant. Cell
Applications
WB, IB, PCA
Huang J, Hume AJ, Abo KM, et al. (2020). SARS-CoV-2 Infection of Pluripotent Stem Cell-derived Human Lung Alveolar Type 2 Cells Elicits a Rapid Epithelial-Intrinsic Inflammatory Response. Cell Stem Cell.
Applications
IF, Confocal Microscopy; FC, FACS, FLOW
Liu Y. et al. (2020). Functional and Genetic Analysis of Viral Receptor ACE2 Orthologs Reveals Broad Potential Host Range of SARS-CoV-2. Proc Natl Acad Sci U S A
Applications
IF, Confocal Microscopy
V’kovski P. et al. (2020). Disparate temperature-dependent virus – host dynamics for SARS-CoV-2 and SARS-CoV in the human respiratory epithelium. PLoS Biol.
Applications
IF, Confocal Microscopy
Nienhold R. et al. (2020). Two distinct immunopathological profiles in autopsy lungs of COVID-19. Nat Commun.
Applications
IHC, ICC, Histology
Grant BD, Anderson CE, Williford JR, et al. (2020). A SARS-CoV-2 Coronavirus Nucleocapsid Antigen-Detecting Half-Strip Lateral Flow Assay Towards the Development of Point of Care Tests Using Commercially Available Reagents. Anal Chem.
Applications
Lateral Flow (LFA)
Daly JL. (2020). Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science
Applications
IF, Confocal Microscopy
Ren W. (2020). Comparative analysis reveals the species-specific genetic determinants of ACE2 required for SARS-CoV-2 entry. PLoS Pathog.
Applications
IF, Confocal Microscopy
Xiaohui Ju et al (2020). A novel cell culture system modeling the SARS-CoV-2 life cycle. PLoS Pathog.
Applications
IF, Confocal Microscopy
Mithal A. et al. (2020). Human Pluripotent Stem Cell Derived Intestinal Organoids Model SARS-CoV-2 Infection Revealing a Common Epithelial Inflammatory Response. Stem Cell Reports.
Applications
Other
Sauvat A et al. (2020). On-target versus off-target effects of drugs inhibiting the replication of SARS-CoV-2. Cell Death & Disease
Applications
WB, IB, PCA
Fattori A. et al. (2020). Cutaneous manifestations in patients with COVID-19: clinical and histological findings. Hum Pathol.
Applications
IHC, ICC, Histology
Cervia C.et al. (2020). Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. Journal of Allergy and Clinical Immunology
Applications
IF, Confocal Microscopy
Hekman R. et al. (2020). Actionable Cytopathogenic Host Responses of Human Alveolar Type 2 Cells to SARS-CoV-2. Mol Cell.
Applications
IF, Confocal Microscopy
Gordon DE et al. (2020). Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science
Applications
Undefined
Chen DY et al. (2020). SARS-CoV-2 desensitizes host cells to interferon through inhibition of the JAK-STAT pathway. bioRxiv Preprint
Applications
IF, Confocal Microscopy
Jossé, L et al. (2019). Experimental determination of codon usage-dependent selective pressure on high copy-number genes in Saccharomyces cerevisiae. Yeast (Chichester, England)
Applications
IF, Confocal Microscopy
THE MATERIALS PROVIDED WITH THIS CERTIFICATE OF ANALYSIS (MATERIALS) ARE FOR RESEARCH USE ONLY AND SUBJECT TO A LIMITED USE LICENSE.
BY USING THIS PRODUCT, THE USER AGREES TO BE BOUND BY THE TERMS OF THE LIMITED USE LICENSE. If the user is not willing to accept the terms of this limited use license and the product is unused, Rockland will accept return of the unused product and provide the user with a full refund. Pursuant to the limited use license, the user may use the product for research use only.
NO TRANSFER OR COMMERCIAL USE OF THIS PRODUCT IS ALLOWED. Commercial use means a use of the product in exchange for consideration, including use in the manufacture of a product, use of the product in the provision of services, and the resale of the product. Rockland reserves all other rights not permitted under the license granted herein, including the rights which are embodied in other products or materials derived from the use of the Materials or know-how embodied in the Materials.
NO RIGHT IS GRANTED UNDER THE LICENSE TO MODIFY OR OTHERWISE CREATE DERIVATIVES OR VARIATIONS OF THE PRODUCT OR TO USE ROCKLAND’S KNOW-HOW OR OTHER INTELLECTUAL PROPERTY FOR OTHER USES. No other use of this product is authorized without the express written prior consent of Rockland, including without limitation, unauthorized commercial uses, in vitro diagnostic uses, therapeutic uses, or any type of consumption by or application to humans or animals.
For uses outside the limited use license, including any diagnostic, therapeutic or commercial use, please contact Rockland for supply and licensing information at licensing@rockland.com.
Products are warranted to operate or perform substantially in conformance with product specifications in effect at the time of shipment, as set forth in the product documentation, specifications and/or package inserts (“Documentation”). The warranty provided is valid only when used by properly trained individuals. Unless otherwise stated in the Documentation, this warranty is limited to one year from the date of shipment when the product is subjected to normal, proper, and intended usage and storage. This warranty does not extend to anyone other than the buyer. No claim of suitability for use in applications regulated by the FDA is made. Any sample furnished to the buyer is merely illustrative of the general type and quality of goods and does not represent that any product will conform to such sample. NO OTHER WARRANTIES, EXPRESS OR IMPLIED, ARE GRANTED, INCLUDING WITHOUT LIMITATION, IMPLIED WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, OR NON-INFRINGEMENT OF THIRD PARTY INTELLECTUAL PROPERTY. BUYER’S EXCLUSIVE REMEDY FOR NON-CONFORMING PRODUCTS DURING THE WARRANTY PERIOD IS LIMITED TO REPAIR, REPLACEMENT, OR REFUND FOR THE NON-CONFORMING PRODUCTS AT SELLER’S SOLE OPTION. There is no obligation to repair, replace, or refund for products as the result of (1) accident, disaster, or event of force majeure, (2) misuse, fault, or negligence of or by the buyer, (3) use of the products in a manner for which they are not designed, or (4) improper storage or handling of the products. In no event shall Rockland be responsible or liable in any way for any third party intellectual property that may address, or is necessary for, use of this product other than as expressly set forth above.
The terms of this limited use license and warranties shall be governed by the laws of the Commonwealth of Pennsylvania.