Datasheet is currently unavailable. Try again or CONTACT US
HSP90 alpha Antibody
Mouse Monoclonal 2G5.G3 IgG
200-301-F69
200 µg
Liquid (sterile filtered)
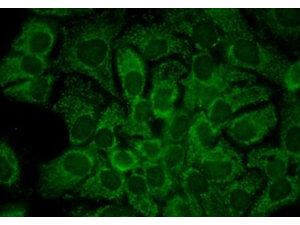
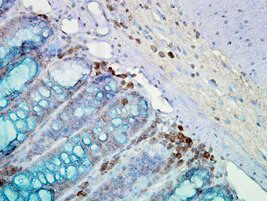
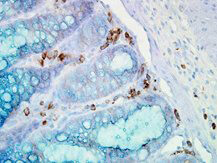
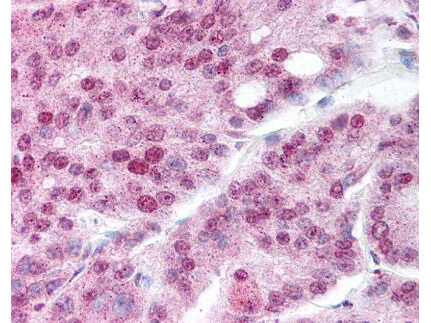
WB, ELISA, IHC, IF, IP
Human, Mouse, Rat
Mouse
Shipping info:
$50.00 to US & $70.00 to Canada for most products. Final costs are calculated at checkout.
Product Details
Anti-HSP90 alpha (MOUSE) Monoclonal Antibody - 200-301-F69
Hsp86, Hsp89A, Hsp90AA1, Hsp90Alpha, HspC1, HSPCA, HspCAL3, Heat shock protein HSP 90-alpha, Heat shock 86 kDa, HSP 86, HSP86, Renal carcinoma antigen NY-REN-38, HSP90AA1, HSP90A, HSPC1, HSPCA
Mouse
Monoclonal
IgG
Target Details
HSP90AA1 - View All HSP90AA1 Products
Human, Mouse, Rat
Recombinant Protein
Hsp90 alpha Antibody was produced in mice by repeated immunizations raised against human Hsp90alpha.
Anti-Hsp90 alpha Antibody was purified by Protein G chromatography. Hsp90α-specific (>96% α-specific by ELISA). A BLAST analysis was used to suggest cross-reactivity with Hsp90α from Human, Mouse, and Rat based on 100% homology with the immunizing sequence. Cross-reactivity with Hsp90 from other sources has not been determined. Heat Shock research.
P07900 - UniProtKB
NP_001017963.2 - NCBI Protein
Application Details
ELISA, IF, IHC, IP, WB
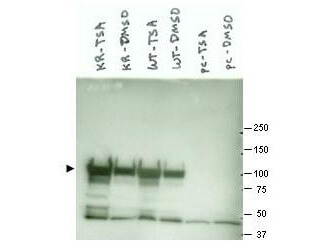
Anti-Hsp90α Antibody is tested for use in WB, IP, ELISA, IF microscopy and IHC. Specific conditions for reactivity should be optimized by the end user.
Formulation
1.0 mg/mL by UV absorbance at 280 nm
0.02 M Potassium Phosphate, 0.15 M Sodium Chloride, pH 7.2
0.09% (w/v) Sodium Azide
50% (v/v) Glycerol
Shipping & Handling
Dry Ice
Store vial at -20° C prior to opening. Aliquot contents and freeze at -20° C or below for extended storage. Avoid cycles of freezing and thawing. Centrifuge product if not completely clear after standing at room temperature. This product is stable for several weeks at 4° C as an undiluted liquid. Dilute only prior to immediate use.
Expiration date is one (1) year from date of receipt.
HSP90 is an abundantly and ubiquitously expressed heat shock protein. It is understood to exist in two principal forms α and β, which share 85% sequence amino acid homology. The two isoforms of Hsp90 are expressed in the cytosolic compartment. Despite the similarities, HSP90α exists predominantly as a homodimer while HSP90β exists mainly as a monomer. From a functional perspective, hsp90 participates in the folding, assembly, maturation, and stabilization of specific proteins as an integral component of a chaperone complex. Furthermore, Hsp90 is highly conserved between species; having 60% and 78% amino acid similarity between mammalian and the corresponding yeast and Drosophila proteins, respectively.
Hsp90 is a highly conserved and essential stress protein that is expressed in all eukaryotic cells. Despite its label of being a heat-shock protein, hsp90 is one of the most highly expressed proteins in unstressed cells (1–2% of cytosolic protein). It carries out a number of housekeeping functions – including controlling the activity, turnover, and trafficking of a variety of proteins. Most of the hsp90-regulated proteins that have been discovered to date are involved in cell signaling. The number of proteins now know to interact with Hsp90 is about 100. Target proteins include the kinases v-Src, Wee1, and c-Raf, transcriptional regulators such as p53 and steroid receptors, and the polymerases of the hepatitis B virus and telomerase. When bound to ATP, Hsp90 interacts with co-chaperones Cdc37, p23, and an assortment of immunophilin-like proteins, forming a complex that stabilizes and protects target proteins from proteasomal degradation.
In most cases, hsp90-interacting proteins have been shown to co-precipitate with hsp90 when carrying out immunoadsorption studies, and to exist in cytosolic heterocomplexes with it. In a number of cases, variations in hsp90 expression or hsp90 mutation has been shown to degrade signaling function via the protein or to impair a specific function of the protein (such as steroid binding, kinase activity) in vivo. Ansamycin antibiotics, such as geldanamycin and radicicol, inhibit hsp90 function.
This product is for research use only and is not intended for therapeutic or diagnostic applications. Please contact a technical service representative for more information. All products of animal origin manufactured by Rockland Immunochemicals are derived from starting materials of North American origin. Collection was performed in United States Department of Agriculture (USDA) inspected facilities and all materials have been inspected and certified to be free of disease and suitable for exportation. All properties listed are typical characteristics and are not specifications. All suggestions and data are offered in good faith but without guarantee as conditions and methods of use of our products are beyond our control. All claims must be made within 30 days following the date of delivery. The prospective user must determine the suitability of our materials before adopting them on a commercial scale. Suggested uses of our products are not recommendations to use our products in violation of any patent or as a license under any patent of Rockland Immunochemicals, Inc. If you require a commercial license to use this material and do not have one, then return this material, unopened to: Rockland Inc., P.O. BOX 5199, Limerick, Pennsylvania, USA.