Datasheet is currently unavailable. Try again or CONTACT US
HSP60 Antibody
Mouse Monoclonal LK2 IgG1
200-301-F61
200 µg
Liquid (sterile filtered)
WB, IHC, FC
Human, Mouse, Rat, Bovine, Chicken, Dog, E. coli (GroEl), Guinea Pig, Hamster, Helicobacter pylori, Monkey, Pig, Rabbit, Salmonella Typhimurium, Spinach, Trichinella spiralis, White Fly, Yeast
Mouse
Shipping info:
$50.00 to US & $70.00 to Canada for most products. Final costs are calculated at checkout.
Product Details
Anti-HSP60 (MOUSE) Monoclonal Antibody - 200-301-F61
CPN60, GROEL, HLD4, Hsp 60, Hsp65, HSPD1, HuCHA60, SPG 13, 60 kDa heat shock protein, mitochondrial, 60 kDa chaperonin, Chaperonin 60, CPN60, Heat shock protein 60, HSP-60, Hsp60, Mitochondrial matrix protein P1, P60 lymphocyte protein, PD1
Mouse
Monoclonal
IgG1
Target Details
HSPD1 - View All HSPD1 Products
Human, Mouse, Rat, Bovine, Chicken, Dog, E. coli (GroEl), Guinea Pig, Hamster, Helicobacter pylori, Monkey, Pig, Rabbit, Salmonella Typhimurium, Spinach, Trichinella spiralis, White Fly, Yeast
Recombinant Protein
Hsp60 Antibody was produced in mice by repeated immunizations raised against recombinant human Hsp60.
Anti-Hsp60 Antibody purified by Protein G chromatography. A BLAST analysis was used to suggest cross-reactivity with Hsp60 from Human, Mouse, Rat, Bovine, Canine, Chicken, Guinea Pig, Hamster, Monkey, Pig, Rabbit, Spinach, E.coli (GroEl), H. pylori, S. typhimurium, T. spiralis , yeast, and white flysources based on 100% homology with the immunizing sequence. Cross-reactivity with Hsp60 from other sources has not been determined. Heat Shock research.
P10809 - UniProtKB
NP_002147.2 - NCBI Protein
Application Details
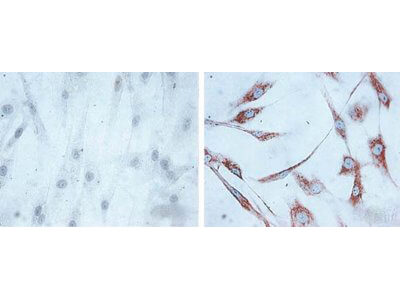
FC, IHC, WB
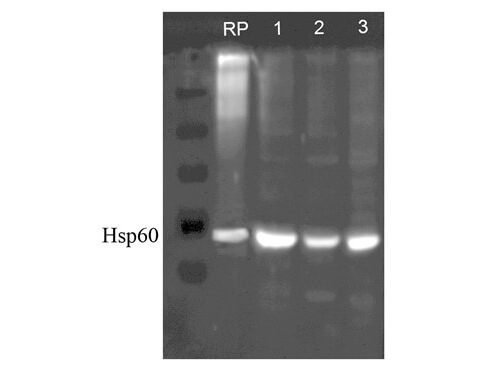
Anti-HSP60 Antibody is tested for WB, Flow Cytometry, and IHC-P. Expect a band approximately ~60kDa protein corresponding to the molecular mass of Hsp60 on SDS PAGE immunoblots. Specific conditions for reactivity should be optimized by the end user. 0.1 mM PMSF added as an inhibitor.
Formulation
1.0 mg/mL by UV absorbance at 280 nm
0.02 M Potassium Phosphate, 0.15 M Sodium Chloride, pH 7.2
0.09% (w/v) Sodium Azide
50% (v/v) Glycerol
Shipping & Handling
Dry Ice
Store vial at -20° C prior to opening. Aliquot contents and freeze at -20° C or below for extended storage. Avoid cycles of freezing and thawing. Centrifuge product if not completely clear after standing at room temperature. This product is stable for several weeks at 4° C as an undiluted liquid. Dilute only prior to immediate use.
Expiration date is one (1) year from date of receipt.
In both prokaryotic and eukaryotic cells, the misfolding and aggregation of proteins during biogenesis and under conditions of cellular stress are prevented by molecular chaperones. Members of the HSP60 family of heat shock proteins are some of the best characterized chaperones. Hsp60, also known as Cpn60 or GroEl, is an abundant protein synthesized constitutively in the cell that is induced to a higher concentration after brief cell shock. It is present in many species and exhibits a remarkable sequence homology among various counterparts in bacteria, plants, and mammals with more than half of the residues identical between bacterial and mammalian Hsp60. Whereas mammalian Hsp60 is localized within the mitochondria, plant Hsp60, or otherwise known as Rubisco-binding protein, is located in plant chloroplasts.
It has been indicated that these proteins carry out a very important biological function due to the fact that Hsp60 is present in so many different species. The common characteristics of the Hsp60s from the divergent species are i) high abundance, ii) induction with environmental stress such as heat shock, iii) homo-oligomeric structures of either 7 or 14 subunits which reversibly dissociate in the presence of Mg2+ and ATP, iv) ATPase activity and v) a role in folding and assembly of oligomeric protein structures. These similarities are supported by recent studies where the single-ring human mitochondrial homolog, Hsp60 with its co-chaperonin, Hsp10 were expressed in a E. coli strain, engineered so that the groE operon is under strict regulatory control. This study has demonstrated that expression of Hsp60-Hsp10 was able to carry out all essential in vivo functions of GroEL and its co-chaperonin, GroES. Another important function of Hsp60 and Hsp10 is their protective functions against infection and cellular stress. Hsp60 has however been linked to a number of autoimmune diseases, as well as Alzheimer´s, coronary artery diseases, MS, and diabetes.
This product is for research use only and is not intended for therapeutic or diagnostic applications. Please contact a technical service representative for more information. All products of animal origin manufactured by Rockland Immunochemicals are derived from starting materials of North American origin. Collection was performed in United States Department of Agriculture (USDA) inspected facilities and all materials have been inspected and certified to be free of disease and suitable for exportation. All properties listed are typical characteristics and are not specifications. All suggestions and data are offered in good faith but without guarantee as conditions and methods of use of our products are beyond our control. All claims must be made within 30 days following the date of delivery. The prospective user must determine the suitability of our materials before adopting them on a commercial scale. Suggested uses of our products are not recommendations to use our products in violation of any patent or as a license under any patent of Rockland Immunochemicals, Inc. If you require a commercial license to use this material and do not have one, then return this material, unopened to: Rockland Inc., P.O. BOX 5199, Limerick, Pennsylvania, USA.