Datasheet is currently unavailable. Try again or CONTACT US
H5N1 Antibody VN04-10
Mouse Monoclonal 8A3 IgG2b kappa
1 References
200-301-978
100 µg
Liquid (sterile filtered)
Neutralization
Virus
Mouse
Shipping info:
$50.00 to US & $70.00 to Canada for most products. Final costs are calculated at checkout.
Product Details
H5N1 Antibody (VN04-10) - 200-301-978
mouse anti-H5N1 Antibody, mouse anti-VN04-10 Antibody, H5HA antibody, Hemagglutinin 5 antibody, H5N1 antibody
Mouse
Monoclonal
IgG2b
Target Details
HA - View All HA Products
Virus
Native Protein
Hemagglutinin of A/Vietnam/1203/04 Influenza Virus (VN04-10) monoclonal antibody was produced by intraperitoneal immunization of BALB/c mice with concentrated purified virus preparation containing hemagglutinin (HA) protein of influenza A virus [strain A/Vietnam/1203/04 (H5N1)] using the modification of the method described by Kohler and Milstein. Each mouse received two immunizations of 15 µg HA with incomplete Freund’s adjuvant, administered 3 week apart.
H5N1 Antibody was purified from tissue culture supernatant fluid by Protein A chromatography and is specific for H5 hemagglutinin (HA) protein of influenza A virus [strain A/Vietnam/1203/04 (H5N1)]. VN04-10 monoclonal antibody did not cross-react with influenza viruses of other HA subtypes. This monoclonal antibody reacted with H5N1 influenza viruses representatives of different clades and subclades of the H5 HA subtype.
Application Details
Neutralization
Hemagglutinin of A/Vietnam/1203/04 Influenza Virus (VN04-10) monoclonal antibody can be used for hemagglutination inhibition (HI) assays to provide antigenic characterization of the influenza A viruses of the H5 HA subtype. This monoclonal antibody is suitable for virus neutralization assays (in cell culture and in embryonated chicken eggs), ELISA, immunoprecipitation, immunohistochemistry and western blotting.
Formulation
1.0 mg/mL by UV absorbance at 280 nm
0.02 M Potassium Phosphate, 0.15 M Sodium Chloride, pH 7.2
0.01% (w/v) Sodium Azide
None
Shipping & Handling
Dry Ice
Store vial at -20° C prior to opening. Aliquot contents and freeze at -20° C or below for extended storage. Avoid cycles of freezing and thawing. Centrifuge product if not completely clear after standing at room temperature. This product is stable for several weeks at 4° C as an undiluted liquid. Dilute only prior to immediate use.
Expiration date is one (1) year from date of receipt.
Hemagglutinin of A/Vietnam/1203/04 Influenza Virus (VN04-10) Antibody raised against the hemagglutinin (HA) surface glycoprotein of the A/Vietnam/1203/04 (H5N1) influenza virus. Generally referred to as ''bird flu'', the H5N1 influenza A virus has been documented in poultry and humans across ten Eurasian countries, from Japan in the north to Indonesia in the south. Without immunity, humans would have no protection against H5N1 influenza viruses, which could potentially cause a catastrophic pandemic influenza. This antibody, directed against the HA surface glycoprotein of the A/Vietnam/1203/04 (H5N1) influenza virus, is intended to further our understanding of the mechanisms underlying antigenic variation and evolution of novel variants. The major functions of HA include receptor-binding and fusion activities, but there may also be a structural role for HA in viral particle formation. Following attachment of HA to surface receptors on susceptible cells, the influenza virus enters the cell via endocytosis and membrane fusion.
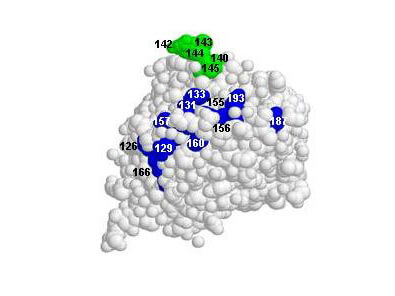
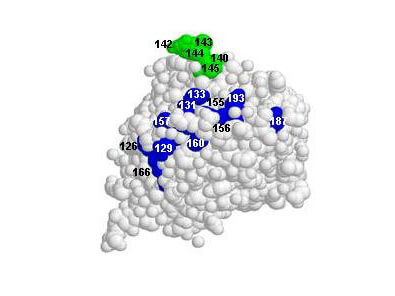
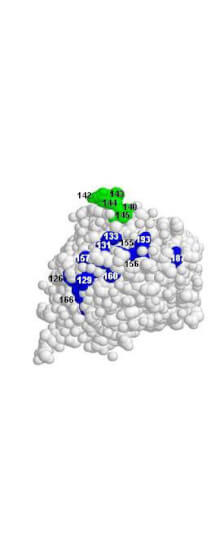
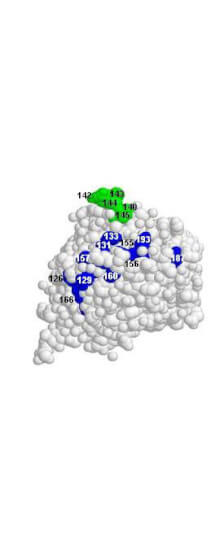
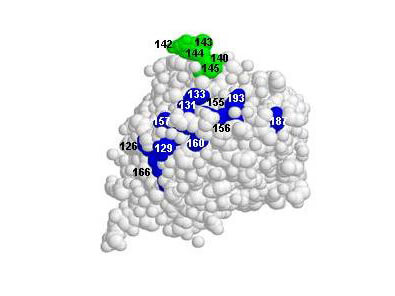
Kaverin NV. et al. (2007). Epitope Mapping of the Hemagglutinin Molecule of a Highly Pathogenic H5N1 Influenza Virus by Using Monoclonal Antibodies. ASM Journals Journal of Virology
Applications
Hemagglutination Inhibition (HI) Assay
This product is for research use only and is not intended for therapeutic or diagnostic applications. Please contact a technical service representative for more information. All products of animal origin manufactured by Rockland Immunochemicals are derived from starting materials of North American origin. Collection was performed in United States Department of Agriculture (USDA) inspected facilities and all materials have been inspected and certified to be free of disease and suitable for exportation. All properties listed are typical characteristics and are not specifications. All suggestions and data are offered in good faith but without guarantee as conditions and methods of use of our products are beyond our control. All claims must be made within 30 days following the date of delivery. The prospective user must determine the suitability of our materials before adopting them on a commercial scale. Suggested uses of our products are not recommendations to use our products in violation of any patent or as a license under any patent of Rockland Immunochemicals, Inc. If you require a commercial license to use this material and do not have one, then return this material, unopened to: Rockland Inc., P.O. BOX 5199, Limerick, Pennsylvania, USA.