Sheep Hemagglutination Kit
KPA-3913
1 Kit
Agglutination
Shipping info:
$50.00 to US & $70.00 to Canada for most products. Final costs are calculated at checkout.
Product Details
Sheep Hemagglutination Kit - KPA-3913
Sheep hemagglutination (HA) assay, Sheep HA kit, Sheep haemagglutination kit, Hemagglutination kit, Sheep hemagglutinins test kit, Lectins hemagglutination assay kit, Virus hemagglutination assay kit, Antibody titer assay kit, Hemagglutination inhibition assay, Haemagglutination-inhibition (HAI) assay kit, HAI assay kit, Sheep HAI kit
Target Details
Anti-SHEEP Red Blood Cell (RBC) (RABBIT) Antibody - 213-4139-0002 is an IgG fraction antibody purified from polyspecific antiserum by a multi-step process which includes delipidation, salt fractionation and ion exchange chromatography followed by extensive dialysis against the buffer stated above. SHEEP RED BLOOD CELLS (RBC) 10% Washed Pooled Cells - R405-0005 are supplied as a 10 percent suspension in phosphate buffered saline (PBS). 10X Phosphate Buffered Saline (PBS) consists of 0.2 M Potassium Phosphate, 1.5 M Sodium Chloride, pH 7.2 prepared in highly polished pharmaceutical grade water (WFI).
Application Details
Agglutination
10X Phosphate Buffered Saline (PBS) is a concentrated stock solution and should be diluted appropriately with distilled, deionized water or equivalent to its final working concentration which is 1X for this kit.
SHEEP RED BLOOD CELLS (RBC) 10% Washed Pooled Cells - R405-0005 should be diluted in 1X PBS to 0.05% concentration.
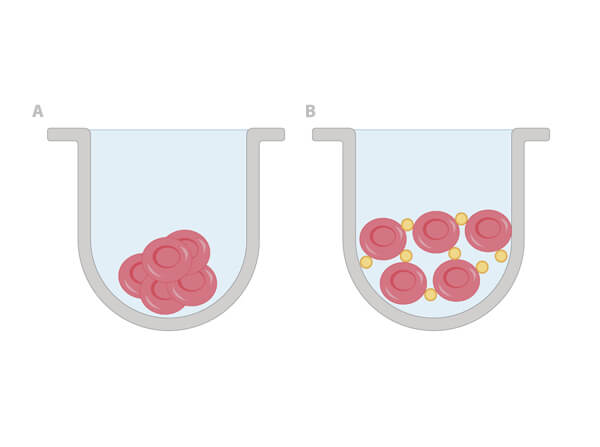
Anti-SHEEP Red Blood Cell (RBC) (RABBIT) Antibody - 213-4139-0002 binds and thus hemagglutinate Sheep RBCs when used at a dilution range of 1:25-1:50. An antibody mediated hemagglutination control is useful as a qualitative index for the integrity of the RBCs in the assay. Further serial dilutions up to 1:400 can be included in the assay if an independent or antibody-based control is required in the assay.
Tissue Data
Mixed
Shipping & Handling
Wet Ice
Store sheep washed pooled red blood cells at 4° C prior to opening. Be advised that blood is a perishable product and exact shelf may depend on application.
This product MAY be stable for up to two (2) weeks if properly stored and handled.
Hemagglutination method is used for titer evaluation of viruses (e.g. Influenza, COVID-19 etc.), bacteria (e.g. Staphylococci, Shigella etc.), proteins (e.g. certain glycoproteins such as Lectins) and other agents which have the ability to attach to red blood cells (RBC)’s surface molecules.
For example, the influenza viruses agglutinate RBCs through their interaction with sialic acid receptors on the host cells. At a molecular level, viral hemagglutinins bind to sialic acid linked to galactose residues through alpha 2.3 or 2.6 linkage, which varies from species to species.
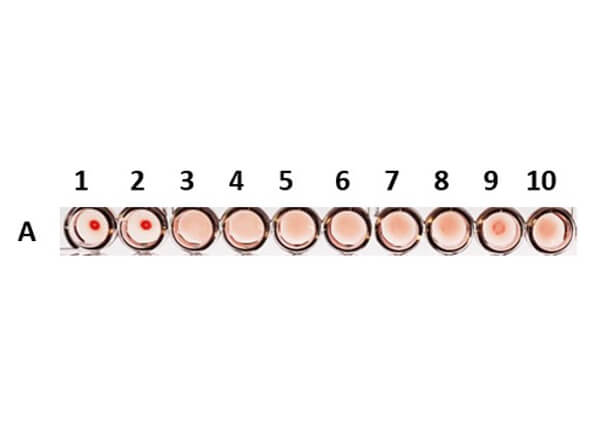
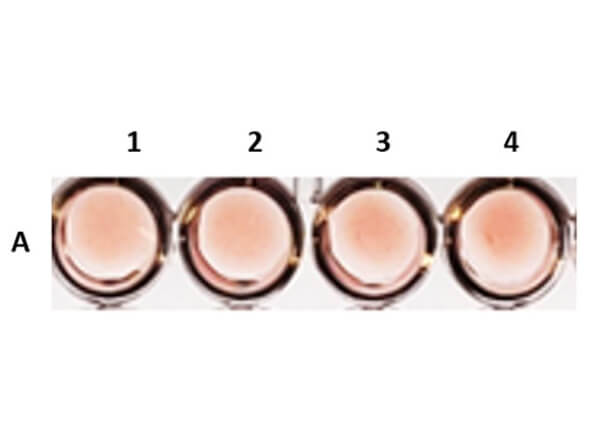
Once a hemagglutinin agglutinates the RBCs, each of the agglutinating molecule attaches to RBCs to form a lattice-structure or a meshwork. This prevents RBCs from settling down from suspension in a round bottom 96 well plate. Visually, a hemagglutinated sample will generate a uniform red colored suspension while a sample lacking hemagglutination will lead to settling down of RBCs to the bottom of wells to form button or dot shape. In viral hemagglutination assay, a virus dilution (e.g. 2-fold from 1:4 to 1:4096) will be applied to an RBC dilution (e.g. 0.05% -0.1%) for 30 minutes to an hour at room temperature or 4° C. Thereafter, lattice formation is observed and the titer of a hemagglutination assay can be determined by the last viable "lattice" structure found.
If diluted further, the amount of Virus particles will be less than the RBCs and thus not be able to agglutinate them.
A Hemagglutination-inhibition (HAI) assay will involve titration of the viral hemagglutination with an anti-viral antibody (often from serum of human or animal infected with that virus) for inhibition of hemagglutination (i.e. neutralization of virus). HAI is one of the most commonly used methods to quantify immunity from influenza and other respiratory viral disease vaccines and is considered the gold standard as a correlate of vaccination mediated protection.
This product is for research use only and is not intended for therapeutic or diagnostic applications. Please contact a technical service representative for more information. All products of animal origin manufactured by Rockland Immunochemicals are derived from starting materials of North American origin. Collection was performed in United States Department of Agriculture (USDA) inspected facilities and all materials have been inspected and certified to be free of disease and suitable for exportation. All properties listed are typical characteristics and are not specifications. All suggestions and data are offered in good faith but without guarantee as conditions and methods of use of our products are beyond our control. All claims must be made within 30 days following the date of delivery. The prospective user must determine the suitability of our materials before adopting them on a commercial scale. Suggested uses of our products are not recommendations to use our products in violation of any patent or as a license under any patent of Rockland Immunochemicals, Inc. If you require a commercial license to use this material and do not have one, then return this material, unopened to: Rockland Inc., P.O. BOX 5199, Limerick, Pennsylvania, USA.