ADA Assay Development
ADA Assay Development
Develop robust immunogenicity assessment strategies with Rockland’s comprehensive anti-drug antibody (ADA) assay development services. Our experts specialize in producing high-quality ADA positive controls to support the accurate detection and quantification of anti-therapeutic immune responses.
With over 25 years of experience partnering with leading biopharma companies, we deliver reliable, fully customized ADA assay solutions that help advance your biologic therapeutics toward regulatory approval and commercial success.
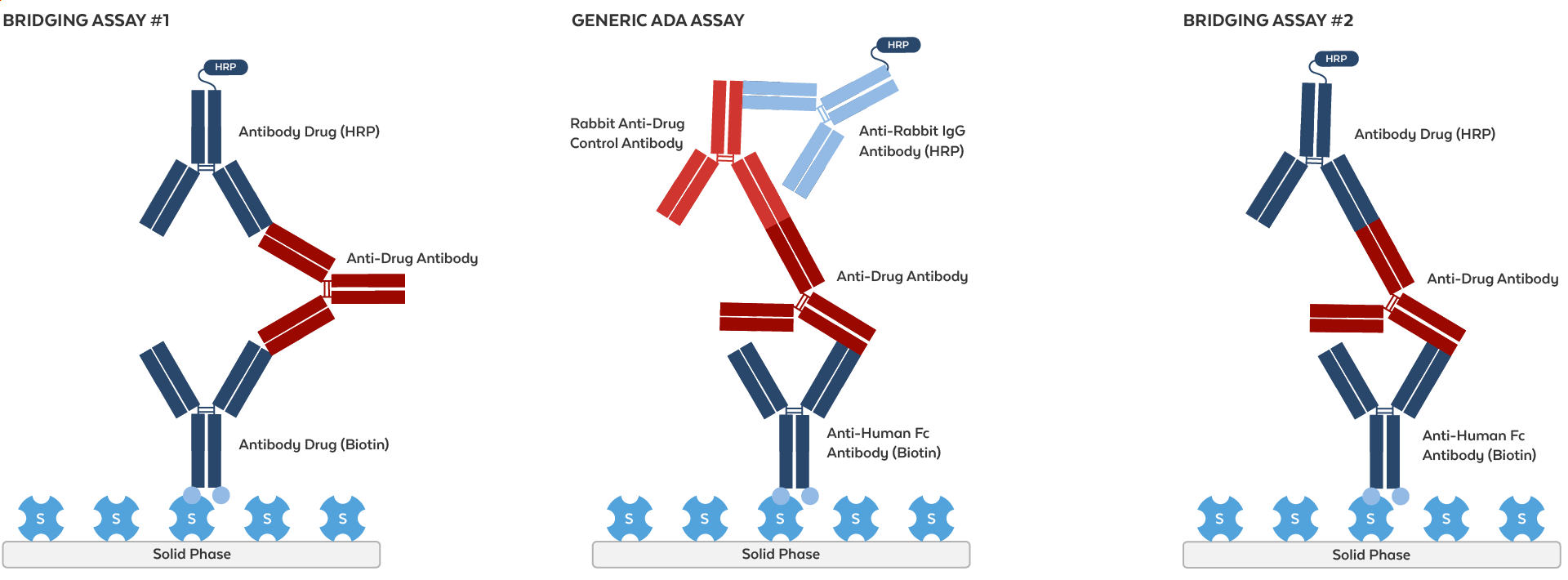
Understanding ADA Assay Formats
ADA assays are designed to detect anti-drug antibodies (ADAs) and can be tailored using various formats depending on the therapeutic and detection requirements.
Our ADA Assay Development Process
Antigen & Immunogen Preparation
We utilize your therapeutic antigen or develop custom immunogens tailored to elicit a robust, specific immune response. Our process ensures the generation of high-affinity anti-drug antibodies capable of detecting subtle differences in drug binding.Anti-Drug Antibody Generation
Using our in-house animal facilities, we raise anti-drug antibodies that serve as ADA positive controls or as assay reagents. Our flexible approach accommodates monoclonal, polyclonal, or recombinant antibody production, depending on your requirements.Purification & Characterization
We employ a range of purification strategies—Protein A, Protein G, Protein A/G, and affinity purification—to achieve the desired specificity and purity levels. Each antibody batch can be tested to confirm its binding characteristics and stability.Conjugation & Customization
To enhance detection capabilities, we offer a variety of conjugation options, including biotin, horseradish peroxidase (HRP), alkaline phosphatase, and a variety of fluorochromes. These customizable reagents help ensure your ADA assays perform optimally in ELISA or other assay platforms.Our Antibody Capabilities
Our expertise extends beyond traditional anti-drug antibody generation. We also specialize in producing various antibody types to support a wide range of therapeutic development programs:
Addressing Immunogenicity in Biologics
Immunogenicity is a critical consideration in the development of biologics, such as therapeutic protein products. Immune responses to these protein-based treatments can significantly influence their pharmacokinetics, pharmacodynamics, safety, and efficacy.
Addressing Immunogenicity in siRNA Therapeutics
As the field of siRNA-based therapies continues to expand, so does the need for rigorous immunogenicity assessments. Anti-drug antibodies (ADAs) have the potential to alter the efficacy, safety, and pharmacokinetics of siRNA drugs, making reliable analytical tools essential for managing these emerging challenges.
Supporting Reagents
To further optimize your ADA assyas, Rockland provides a wide range of supporting reagents that enhance assay performance, including detection antibodies, substrates, stablizers, and bocking buffers:
| Product | Usage |
| Human IgG Fc Antibody | Capture & Detection |
| Human IgG Fc Antibody Biotin Conjugated | Capture |
| Chemiluminescent FemtoMax™ Super Sensitive HRP Substrate | Substrate |
| TMB ELISA Peroxidase Substrate | Substrate |
| TMB Prestained Red ELISA Peroxidase Substrate | Substrate |
| ELISA Coating Stabilizer | Stabilizer |
| HRP Conjugate Stabilizer | Stabilizer |
| ELISA Microwell Blocking Buffer with Stabilizer | Stabilizer + Blocking Buffer |
| Blocking Buffer for Fluorescent Western Blotting | Blocking Buffer |
| Blocking Buffer (2X) for Fluorescent Western Blotting | Blocking Buffer |
| 10X BBS Fish Gel Concentrate | Blocking Buffer |
| 10X PBS Fish Gel Concentrate | Blocking Buffer |
| 10X TBS Fish Gel Concentrate | Blocking Buffer |
Interested in ADA assay development services?
We can help move your program forward—contact us to find out how.